Immunomodulatory Activity of Polysaccharides Isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb
Abstract
:1. Introduction
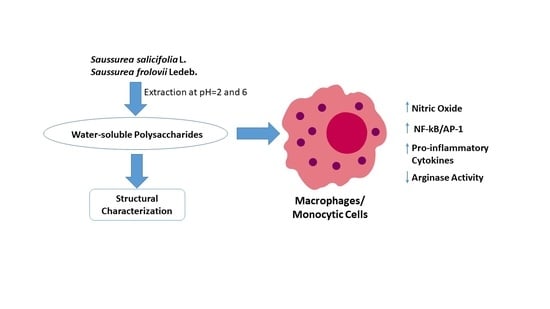
2. Results and Discussion
2.1. Partial Characterization of Saussurea Polysaccharides
2.2. Effects of Saussurea Polysaccharides on NO Production in Mouse Macrophages
2.3. Effects of Saussurea Polysaccharides on Arginase Activity in Mouse Macrophages
2.4. Effects of Saussurea Polysaccharides on AP-1/NF-κB Transcriptional Activity
2.5. Effects of Saussurea Polysaccharides on Cytokine Production
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Fractionation of Saussurea Polysaccharides
3.3. Characterization of Saussurea Polysaccharide Fractions
3.4. Cell Culture
3.5. Analysis of Macrophage Nitric Oxide (NO) Production
3.6. Arginase Assay
3.7. Analysis of AP-1/NF-κB Activation
3.8. Cytokine Analysis
3.9. Cell Viability Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chik, W.I.; Zhu, L.; Fan, L.L.; Yi, T.; Zhu, G.Y.; Gou, X.J.; Tang, Y.N.; Xu, J.; Yeung, W.P.; Zhao, Z.Z.; et al. Saussurea involucrata: A review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J. Ethnopharmacol. 2015, 172, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Ni, Z.Y.; Dong, M.; Cong, B.; Shi, Q.W.; Gu, Y.C.; Kiyota, H. Secondary metabolites of plants from the genus Saussurea: Chemistry and biological activity. Chem. Biodiver. 2010, 7, 2623–2659. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, E.; Reshetov, Y.; Domrachev, D.; Gulina, E.; Krivoshchekov, S.; Shurupova, M.; Brazovskii, K.; Belousov, M. Constituent composition of the essential oils from some species of the genus Saussurea DC. Nat. Prod. Res. 2022, 36, 660–663. [Google Scholar] [CrossRef]
- Maqsood, S.; Miana, G.A.; Kanwal, M.; Malik, S.; Saleem, S.; Maqsood, A.; Rehman, H.; Hayat, S.; Farooq, M.U. Phytochemical investigation and pharmacological evaluation of methanolic flower extract of Saussurea heteromalla. Pakistan J. Pharmaceut. Sci. 2022, 35, 1153–1158. [Google Scholar]
- Naseer, S.; Iqbal, J.; Naseer, A.; Kanwal, S.; Hussain, I.; Tan, Y.; Aguilar-Marcelino, L.; Cossio-Bayugar, R.; Zajac, Z.; Bin Jardan, Y.A.; et al. Deciphering chemical profiling, pharmacological responses and potential bioactive constituents of Saussurea lappa Decne. Extracts through in vitro approaches. Saudi J. Biol. Sci. 2022, 29, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.M.; Wu, C.F.; Liu, W.; Yu, H.; Hao, Y.; Zheng, J.H.; Ji, Y.R. Antiinflammatory and analgesic activities of the tissue culture of Saussurea involucrata. Biol. Pharmaceut. Bull. 2005, 28, 1612–1614. [Google Scholar] [CrossRef]
- Kamilijiang, M.; Zang, D.; Abudukelimu, N.; Aidarhan, N.; Liu, G.Y.; Aisa, H.A. Anti-melanogenesis effect of polysaccharide from Saussurea involucrata on forskolin-induced melanogenesis in B16F10 melanoma cells. Nutrients 2022, 14, 5044. [Google Scholar] [CrossRef]
- Chen, W.B.; Zhu, X.L.; Ma, J.J.; Zhang, M.M.; Wu, H. Structural elucidation of a novel pectin-polysaccharide from the petal of Saussurea laniceps and the mechanism of its anti-HBV activity. Carbohydr. Polym. 2019, 223, 115077. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, J.; Ye, J.; Ma, W.Y.; Yan, H.L.; Wang, G. Saussurea tridactyla Sch Bip.-derived polysaccharides and flavones reduce oxidative damage in ultraviolet B-irradiated HaCaT cells via a p38MAPK-independent mechanism. Drug Des. Dev. Ther. 2016, 10, 389–403. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Tosi, M.F. Innate immune responses to infection. J. Allergy Clin. Immunol. 2005, 116, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Birk, R.W.; Gratchev, A.; Hakiy, N.; Politz, O.; Schledzewski, K.; Guillot, P.; Orfanos, C.E.; Goerdt, S. Alternatively activated antingen presenting cells. Basic concepts and clinical relevance. Hautarzt 2001, 52, 193–200. [Google Scholar] [CrossRef]
- Lingen, M.W. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch. Pathol. Lab. Med. 2001, 125, 67–71. [Google Scholar] [CrossRef]
- Klimp, A.H.; de Vries, E.G.E.; Scherphof, G.L.; Daemen, T. A potential role of macrophage activation in the treatment of cancer. Crit. Rev. Oncol. Hemat. 2002, 44, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.B.; Hancock, R.E.W. Opinion—Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2004, 2, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Lv, Q.Q.; Zhang, B.; Chen, H.Q. Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem. Carbohyd. Polym. 2019, 212, 89–101. [Google Scholar] [CrossRef]
- Deng, W.W.; Yang, X.; Zhu, Y.; Yu, J.N.; Xu, X.M. Structural characterization and hypolipidemic activities of purified Stigma maydis polysaccharides. Food Sci. Nut. 2019, 7, 2674–2683. [Google Scholar] [CrossRef]
- Liu, G.; Kamilijiang, M.; Abuduwaili, A.; Zang, D.; Abudukelimu, N.; Liu, G.; Yili, A.; HA, A.I. Isolation, structure elucidation, and biological activity of polysaccharides from Saussurea involucrata. Int. J. Biol. Macromol. 2022, 222, 154–166. [Google Scholar] [CrossRef]
- Yang, Z.H.; Ming, X.F. Arginase: The emerging therapeutic target for vascular oxidative stress and inflammation. Front. Immunol. 2013, 4, 149. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nature Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Kim, M.J.; Kang, S.; Moon, N.R.; Kim, D.S.; Lee, N.R.; Kim, K.S.; Park, S. Topical treatments of Saussurea costus root and Thuja orientalis L. synergistically alleviate atopic dermatitis-like skin lesions by inhibiting protease-activated receptor-2 and NF-κB signaling in HaCaT cells and Nc/Nga mice. J. Ethnopharmacol. 2017, 199, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Su, D.; Xian, X.Y.; Zhou, M.Y.; Li, X.Z.; Huang, J.; Wang, J.H.; Gao, H.Y. Inhibitory effects of Saussurea involucrata (Kar. et Kir.) Sch -Bip. on adjuvant arthritis in rats. J. Ethnopharmacol. 2016, 194, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Enbergs, H. Effects of Saussurea lappa roots extract in ethanol on leukocyte phagocytic activity, lymphocyte proliferation and interferon-g (IFN-g). Pakistan J. Pharmaceut. Sci. 2007, 20, 175–179. [Google Scholar]
- Cong, X.Y.; He, J.Y.; Shu, T.Y.; Chen, H.; Feng, Y.; Su, L.H.; Xu, M. Undescribed amino acid-sesquiterpene lactone adducts and sesquiterpene glycosides from the roots of Saussurea lappa and their anti-HBV activity. Fitoterapia 2023, 169, 105570. [Google Scholar] [CrossRef]
- Zhou, K.; Taoerdahong, H.; Bai, J.; Bakasi, A.; Wang, X.; Dong, C. Structural characterization and immunostimulatory activity of polysaccharides from Pyrus sinkiangensis Yu. Int. J. Biol. Macromol. 2020, 157, 444–451. [Google Scholar] [CrossRef]
- Martins, V.M.R.; Simões, J.; Ferreira, I.; Cruz, M.T.; Domingues, M.R.; Coimbra, M.A. In vitro macrophage nitric oxide production by Pterospartum tridentatum (L.) Willk. inflorescence polysaccharides. Carbohydr. Polym. 2017, 157, 176–184. [Google Scholar] [CrossRef]
- Tang, Y.; He, X.; Liu, G.; Wei, Z.; Sheng, J.; Sun, J.; Li, C.; Xin, M.; Li, L.; Yi, P. Effects of different extraction methods on the structural, antioxidant and hypoglycemic properties of red pitaya stem polysaccharide. Food Chem. 2023, 405, 134804. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Ji, Z.; He, L.; Regev, A.; Struhl, K. Inflammatory regulatory network mediated by the joint action of NF-kB, STAT3, and AP-1 factors is involved in many human cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9453–9462. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, M.A.; Pasco, D.S. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 2001, 49, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Hu, Q.; Chang, Z.H.; Liu, Y.Q.; Gao, Y.; Luo, X.W.; Zhou, L.P.; Chen, Y.X.; Cui, Y.T.; Wang, Z.H.; et al. Moringa oleifera leaf polysaccharides exert anti-lung cancer effects upon targeting TLR4 to reverse the tumor-associated macrophage phenotype and promote T-cell infiltration. Food Funct. 2023, 14, 4607–4620. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Yelithao, K.; Palanisamy, S.; Prabhu, N.M.; Nan, M. Isolation, structural elucidation and immuno-stimulatory properties of polysaccharides from Cuminum cyminum. Carbohydr. Polym. 2020, 230, 115636. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, M.; Hao, H.; Deng, J.; Li, M.; Sun, Y.; Yang, R.; Wang, H.; Huang, R. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. Int. J. Biol. Macromol. 2019, 136, 324–331. [Google Scholar] [CrossRef]
- Ye, J.; Hua, X.; Wang, M.; Zhang, W.; Yang, R. Effect of extraction pH on the yield and physicochemical properties of polysaccharides extracts from peanut sediment of aqueous extraction process. LWT 2019, 106, 137–144. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Scott, R.W. Colorimetric determination of hexuronic acids in plant materials. Analyt. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.B.; Ge, J.C.; Zhang, W.J.; Liu, W.; Luo, J.P.; Xu, F.Q.; Wu, D.L.; Xie, S.Z. Physicochemical, morpho-structural, and biological characterization of polysaccharides from three Polygonatum spp. RSC Adv. 2021, 11, 37952–37965. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernandez-Hernandez, O.; Rodriguez-Sanchez, S.; Sanz, M.L.; Martinez-Castro, I. Derivatization of carbohydrates for GC and GC-MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Schepetkin, I.A.; Quinn, M.T. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int. Immunopharmacol. 2007, 7, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kouakou, K.; Yapi, A.; Kirpotina, L.N.; Jutila, M.A.; Quinn, M.T. Immunomodulatory and hemagglutinating activities of acidic polysaccharides isolated from Combretum racemosum. Int. Immunopharmacol. 2013, 15, 628–637. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kovrizhina, A.R.; Stankevich, K.S.; Khlebnikov, A.I.; Kirpotina, L.N.; Quinn, M.T.; Cook, M.J. Design, synthesis and biological evaluation of novel O-substituted tryptanthrin oxime derivatives as c-Jun N-terminal kinase inhibitors. Front. Pharmacol. 2022, 13, 958687. [Google Scholar] [CrossRef] [PubMed]





| Fraction | SSP2 | SSP6 | SFP2 | SFP6 |
|---|---|---|---|---|
| Yield (%) | 1.13 ± 0.16 | 1.82 ± 0.10 * | 1.50 ± 0.23 | 2.71 ± 0.11 * |
| Hexose (%) | 44. 04 ± 3.53 | 33.88 ± 1.03 * | 33.55 ± 5.77 | 23.98 ± 2.46 |
| Uronic Acid (%) | 7.71 ± 0.75 | 3.08 ± 0.42 * | 4.76 ± 1.13 | 1.40 ± 0.27 * |
| O-acetyl group (μM/mL) | 1.44 ± 0.05 | 2.30 ± 0.44 * | 1.67 ± 0.24 | 1.61 ± 0.19 |
| Protein (%) | 6.51 ± 1.43 | 33.86 ± 6.78 * | 13.65 ± 2.10 | 13.78 ± 1.54 |
| M.W. (kDa) | 143.66 ± 19.01 | 113.16 ± 16.64 | 75.29 ± 10.30 | 64.27 ± 6.55 |
| Fraction | SSP2 | SSP6 | SFP2 | SFP6 |
|---|---|---|---|---|
| Glu | 11.8 ± 0.2 | 19.2 ± 0.4 * | 32.9 ± 0.6 | 28.3 ± 0.5 * |
| Gal | 4.7 ± 0.1 | 8.3 ± 0.1 * | N.F. | N.F. |
| Xyl | 76.7 ± 0.9 | 63.1 ± 0.8 * | 67.1 ± 0.7 | 71.7 ± 0.7 * |
| Rha | 6.8 ± 0.1 | 9.4 ± 0.1 * | N.F. | N.F. |
| Ara | N.F. | N.F. | N.F. | N.F. |
| Man | N.F. | N.F. | N.F. | N.F. |
| Test Sample | Arginase Activity (E.U.) |
|---|---|
| SSP2 (20 μg/mL) | 42.26 ± 0.43 * |
| SSP6 (20 μg/mL) | 39.55 ± 0.64 * |
| SFP2 (20 μg/mL) | 37.15 ± 0.64 *# |
| LPS (100 ng/mL) | 45.67 ± 0.58 * |
| Control (media along) | 53.94 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Danilets, M.G.; Ligacheva, A.A.; Trofimova, E.S.; Selivanova, N.S.; Sherstoboev, E.Y.; Krivoshchekov, S.V.; Gulina, E.I.; Brazovskii, K.S.; Kirpotina, L.N.; et al. Immunomodulatory Activity of Polysaccharides Isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb. Molecules 2023, 28, 6655. https://doi.org/10.3390/molecules28186655
Schepetkin IA, Danilets MG, Ligacheva AA, Trofimova ES, Selivanova NS, Sherstoboev EY, Krivoshchekov SV, Gulina EI, Brazovskii KS, Kirpotina LN, et al. Immunomodulatory Activity of Polysaccharides Isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb. Molecules. 2023; 28(18):6655. https://doi.org/10.3390/molecules28186655
Chicago/Turabian StyleSchepetkin, Igor A., Marina G. Danilets, Anastasia A. Ligacheva, Evgenia S. Trofimova, Natalia S. Selivanova, Evgenii Yu. Sherstoboev, Sergei V. Krivoshchekov, Ekaterina I. Gulina, Konstantin S. Brazovskii, Liliya N. Kirpotina, and et al. 2023. "Immunomodulatory Activity of Polysaccharides Isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb" Molecules 28, no. 18: 6655. https://doi.org/10.3390/molecules28186655
APA StyleSchepetkin, I. A., Danilets, M. G., Ligacheva, A. A., Trofimova, E. S., Selivanova, N. S., Sherstoboev, E. Y., Krivoshchekov, S. V., Gulina, E. I., Brazovskii, K. S., Kirpotina, L. N., Quinn, M. T., & Belousov, M. V. (2023). Immunomodulatory Activity of Polysaccharides Isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb. Molecules, 28(18), 6655. https://doi.org/10.3390/molecules28186655