Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells
Abstract
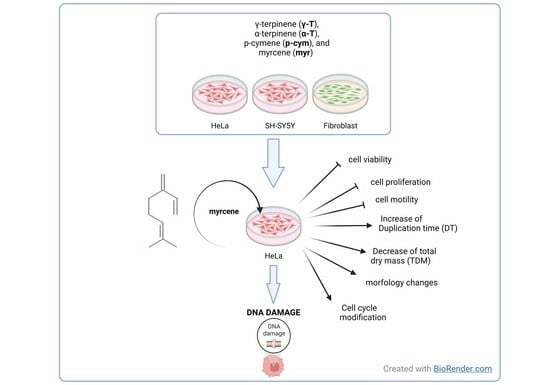
:1. Introduction
2. Results and Discussion
2.1. Cell Viability
2.2. Effects on Cell Proliferation, Motility, and Morphology
2.3. Myrcene Induced DNA Damage in HeLa Cells
3. Materials and Methods
3.1. Cell Culture and Treatments
3.1.1. MTT Assay
3.1.2. Quantitative Phase Image Microscopy
3.1.3. Cell-Cycle and Apoptosis Analysis Using Flow Cytometry
3.1.4. Histone Post-Translational Modification
3.1.5. DNA–Compound Interaction Assay
3.2. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Elmadfa, I. Biological Relevance of Terpenoids. Ann. Nutr. Metab. 2003, 47, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L. Prevention and Therapy of Cancer by Dietary Monoterpenes. J. Nutr. 1999, 129, 775S–778S. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.N. Cancer Chemoprevention and Therapy by Monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar] [CrossRef]
- Silva, B.I.M.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer Activity of Monoterpenes: A Systematic Review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Graßmann, J.; Schneider, D.; Weiser, D.; Elstner, E. Antioxidative Effects of Lemon Oil and Its Components on Copper Induced Oxidation of Low Density Lipoprotein. Arzneimittelforschung 2011, 51, 799–805. [Google Scholar] [CrossRef]
- Takahashi, Y.; Inaba, N.; Kuwahara, S.; Kuki, W. Antioxidative Effect of Citrus Essential Oil Components on Human Low-Density Lipoprotein In Vitro. Biosci. Biotechnol. Biochem. 2003, 67, 195–197. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of Lipid Peroxidation by γ-Terpinene, an Unusual and Potentially Useful Hydrocarbon Antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef]
- Milde, J.; Elstner, E.F.; Graßmann, J. Synergistic Inhibition of Low-Density Lipoprotein Oxidation by Rutin, γ-Terpinene, and Ascorbic Acid. Phytomedicine 2004, 11, 105–113. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; De Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The Progress of Essential Oils as Potential Therapeutic Agents: A Review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative Study of the Antitumor Effect of Natural Monoterpenes: Relationship to Cell Cycle Analysis. Rev. Bras. Farmacogn. 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Baschieri, A.; Jin, Z.; Amorati, R. Hydroperoxyl Radical (HOO•) as a Reducing Agent: Unexpected Synergy with Antioxidants. A Review. Free Radic. Res. 2023, 57, 115–129. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.C.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.P.; da Silva, T.B.; Machado, W.J.; Prata, A.P.N.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; et al. Cytotoxic Effect of Leaf Essential Oil of Lippia Gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Döll-Boscardin, P.M.; Sartoratto, A.; Sales Maia, B.H.L.d.N.; Padilha de Paula, J.; Nakashima, T.; Farago, P.V.; Kanunfre, C.C. In Vitro Cytotoxic Potential of Essential Oils of Eucalyptus benthamii and Its Related Terpenes on Tumor Cell Lines. Evid.-Based Complement. Altern. Med. 2012, 2012, 342652. [Google Scholar] [CrossRef]
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of Black Cumin Essential Oil and Its Main Terpenes from Tunisia. S. Afr. J. Bot. 2010, 76, 210–216. [Google Scholar] [CrossRef]
- Assmann, C.E.; Cadoná, F.C.; Bonadiman, B.D.S.R.; Dornelles, E.B.; Trevisan, G.; Cruz, I.B.M.D. Tea Tree Oil Presents in Vitro Antitumor Activity on Breast Cancer Cells without Cytotoxic Effects on Fibroblasts and on Peripheral Blood Mononuclear Cells. Biomed. Pharmacother. 2018, 103, 1253–1261. [Google Scholar] [CrossRef]
- Jin, H.; Leng, Q.; Zhang, C.; Zhu, Y.; Wang, J. P-Cymene Prevent High-Fat Diet-Associated Colorectal Cancer by Improving the Structure of Intestinal Flora. J. Cancer 2021, 12, 4355–4361. [Google Scholar] [CrossRef]
- Pujante-Galián, M.A.; Pérez, S.A.; Montalbán, M.G.; Carissimi, G.; Fuster, M.G.; Víllora, G.; García, G. P-Cymene Complexes of Ruthenium(II) as Antitumor Agents. Molecules 2020, 25, 5063. [Google Scholar] [CrossRef]
- Chaouki, W.; Leger, D.Y.; Liagre, B.; Beneytout, J.-L.; Hmamouchi, M. Citral Inhibits Cell Proliferation and Induces Apoptosis and Cell Cycle Arrest in MCF-7 Cells. Fundam. Clin. Pharmacol. 2009, 23, 549–556. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Bai, X.; Tang, J. Myrcene Exhibits Antitumor Activity Against Lung Cancer Cells by Inducing Oxidative Stress and Apoptosis Mechanisms. Nat. Prod. Commun. 2020, 15, 1934578X2096118. [Google Scholar] [CrossRef]
- Silva, S.L.D.; Figueiredo, P.M.; Yano, T. Cytotoxic Evaluation of Essential Oil from Zanthoxylum Rhoifolium Lam. Leaves. Acta Amaz. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Hwang, E.; Ngo, H.T.T.; Park, B.; Seo, S.-A.; Yang, J.-E.; Yi, T.-H. Myrcene, an Aromatic Volatile Compound, Ameliorates Human Skin Extrinsic Aging via Regulation of MMPs Production. Am. J. Chin. Med. 2017, 45, 1113–1124. [Google Scholar] [CrossRef]
- Cadart, C.; Monnier, S.; Grilli, J.; Sáez, P.J.; Srivastava, N.; Attia, R.; Terriac, E.; Baum, B.; Cosentino-Lagomarsino, M.; Piel, M. Size Control in Mammalian Cells Involves Modulation of Both Growth Rate and Cell Cycle Duration. Nat. Commun. 2018, 9, 3275. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.-Y.; Lin, J.; Amir, A. Modeling Cell Size Regulation: From Single-Cell-Level Statistics to Molecular Mechanisms and Population-Level Effects. Annu. Rev. Biophys. 2018, 47, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.C. The Regulation of Cell Size. Cell 2013, 154, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.P.; Kang, J.H.; Yang, L.F.; Manalis, S.R. Mammalian Cell Growth Dynamics in Mitosis. eLife 2019, 8, e44700. [Google Scholar] [CrossRef] [PubMed]
- Schmoller, K.M.; Skotheim, J.M. The Biosynthetic Basis of Cell Size Control. Trends Cell Biol. 2015, 25, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Lehmann, R. Mechanisms Guiding Primordial Germ Cell Migration: Strategies from Different Organisms. Nat. Rev. Mol. Cell Biol. 2010, 11, 37–49. [Google Scholar] [CrossRef] [PubMed]
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in Mesoderm, Neural Tube and Vascular Development in Mouse Embryos Lacking Fibronectin. Development 1993, 119, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Grose, R.; Werner, S.; Kessler, D.; Tuckermann, J.; Huggel, K.; Durka, S.; Reichardt, H.M.; Werner, S. A Role for Endogenous Glucocorticoids in Wound Repair. EMBO Rep. 2002, 3, 575–582. [Google Scholar] [CrossRef]
- Martin, P.; Parkhurst, S.M. Parallels between Tissue Repair and Embryo Morphogenesis. Development 2004, 131, 3021–3034. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-Cell Invasion and Migration: Diversity and Escape Mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Bacac, M.; Stamenkovic, I. Metastatic Cancer Cell. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 221–247. [Google Scholar] [CrossRef]
- Schlegel, R.A.; Williamson, P. PS to PS (Phosphatidylserine)–Pertinent Proteins in Apoptotic Cell Clearance. Sci. STKE 2007, 2007, pe57. [Google Scholar] [CrossRef]
- Zhou, Z. New Phosphatidylserine Receptors: Clearance of Apoptotic Cells and More. Dev. Cell 2007, 13, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Zwaal, R.F.A.; Comfurius, P.; Bevers, E.M. Surface Exposure of Phosphatidylserine in Pathological Cells. CMLS Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Van Genderen, H.; Kenis, H.; Lux, P.; Ungeth, L.; Maassen, C.; Deckers, N.; Narula, J.; Hofstra, L.; Reutelingsperger, C. In Vitro Measurement of Cell Death with the Annexin A5 Affinity Assay. Nat. Protoc. 2006, 1, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, T.H.; Gorovsky, M.A. Phylogenetic Analysis of the Core Histones H2A, H2B, H3, and H4. Nucleic Acids Res. 1994, 22, 174–179. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.-X. Gamma-H2AX—A Novel Biomarker for DNA Double-Strand Breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Sherr, C.J. The Pezcoller Lecture: Cancer Cell Cycles Revisited. Cancer Res. 2000, 60, 3689–3695. [Google Scholar]
- Senderowicz, A.M. Preclinical and Clinical Development of Cyclin-Dependent Kinase Modulators. J. Natl. Cancer Inst. 2000, 92, 376–387. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular Processing of Platinum Anticancer Drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Micheletti, G.; Calonghi, N.; Farruggia, G.; Strocchi, E.; Palmacci, V.; Telese, D.; Bordoni, S.; Frisco, G.; Boga, C. Synthesis of Novel Structural Hybrids between Aza-Heterocycles and Azelaic Acid Moiety with a Specific Activity on Osteosarcoma Cells. Molecules 2020, 25, 404. [Google Scholar] [CrossRef]
- De Nisi, A.; Bergamini, C.; Leonzio, M.; Sartor, G.; Fato, R.; Naldi, M.; Monari, M.; Calonghi, N.; Bandini, M. Synthesis, Cytotoxicity and Anti-Cancer Activity of New Alkynyl-Gold (I) Complexes. Dalton Trans. 2016, 45, 1546–1553. [Google Scholar] [CrossRef] [PubMed]










| Compound | Kd ± SE (μM) | r2 |
|---|---|---|
| γ-T | 74 ± 83 | 0.8316 |
| α-T | 17 ± 22 | 0.7311 |
| p-cym | 493 ± 4425 | 0.6339 |
| myr | 29 ± 18 | 0.9360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pincigher, L.; Valenti, F.; Bergamini, C.; Prata, C.; Fato, R.; Amorati, R.; Jin, Z.; Farruggia, G.; Fiorentini, D.; Calonghi, N.; et al. Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells. Molecules 2023, 28, 6728. https://doi.org/10.3390/molecules28186728
Pincigher L, Valenti F, Bergamini C, Prata C, Fato R, Amorati R, Jin Z, Farruggia G, Fiorentini D, Calonghi N, et al. Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells. Molecules. 2023; 28(18):6728. https://doi.org/10.3390/molecules28186728
Chicago/Turabian StylePincigher, Luca, Francesca Valenti, Christian Bergamini, Cecilia Prata, Romana Fato, Riccardo Amorati, Zongxin Jin, Giovanna Farruggia, Diana Fiorentini, Natalia Calonghi, and et al. 2023. "Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells" Molecules 28, no. 18: 6728. https://doi.org/10.3390/molecules28186728
APA StylePincigher, L., Valenti, F., Bergamini, C., Prata, C., Fato, R., Amorati, R., Jin, Z., Farruggia, G., Fiorentini, D., Calonghi, N., & Zalambani, C. (2023). Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells. Molecules, 28(18), 6728. https://doi.org/10.3390/molecules28186728