Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives
Abstract
:1. Introduction
2. Beneficial Bacteria and Crop Disease Management
3. Beneficial Fungi and Crop Diseases Management
4. Biocontrol Mechanisms in Controlling Plant Diseases
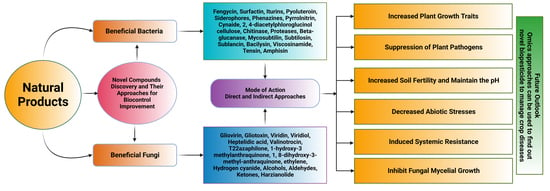
4.1. Microbial Natural Products: A Potential Weapon in the Agriculture Sector
4.2. Bacteria as a Valuable Source of Natural Products
4.3. Fungal Natural Products for Crop Disease Prevention
4.4. Competition of Biocontrol Agents with Other Rhizosphere Microbes
4.5. Biocontrol Agents Promote Plant Growth
5. Factors Affecting Biocontrol of Plant Diseases and Selection of Potential BCAs
5.1. Plant Species Influence the Biocontrol Activity of BCAs
5.2. Pathogen Influence the Biocontrol Activity of BCAs
5.3. Biocontrol Agents and Their Specific Nature
5.4. Environmental Stresses Impact on BCA Activity
6. Challenges in Establishing Beneficial Microbes as BCAs
6.1. The Journey of Biocontrol Agents from Lab to Field
6.2. Limited Number of Registered Biopesticides and Lack of Awareness
6.3. Biopesticides Commercialization and Legislative Procedure
7. Biotechnological and Omics Techniques for Biocontrol Strategy Improvement
7.1. Biotechnological Techniques Linked with Biocontrol Strategy
7.2. Biocontrol Strategy in the Era of Multi-Omics
7.3. Microbiome-Based Solution for Plant Disease Management
7.4. Microbiome Engineering: A Shining Approach in Biocontrol Strategy
8. Research Gaps and Future Direction
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus Strain S2 Shows High Nematicidal Activity against Meloidogyne incognita by Producing Sphingosine. Sci. Rep. 2016, 6, 28756. [Google Scholar] [CrossRef] [PubMed]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile Compounds of Endophytic Bacillus spp. Have Biocontrol Activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef]
- Zubair, M.; Farzand, A.; Mumtaz, F.; Khan, A.R.; Sheikh, T.M.M.; Haider, M.S.; Yu, C.; Wang, Y.; Ayaz, M.; Gu, Q.; et al. Novel Genetic Dysregulations and Oxidative Damage in Fusarium graminearum Induced by Plant Defense Eliciting Psychrophilic Bacillus atrophaeus Ts1. Int. J. Mol. Sci. 2021, 22, 12094. [Google Scholar] [CrossRef]
- Schoina, C.; Stringlis, I.A.; Pantelides, I.S.; Tjamos, S.E.; Paplomatas, E.J. Evaluation of Application Methods and Biocontrol Efficacy of Paenibacillus alvei Strain K-165, against the Cotton Black Root Rot Pathogen Thielaviopsis basicola. Biol. Control 2011, 58, 68–73. [Google Scholar] [CrossRef]
- Zaker, M. Natural Plant Products as Eco-Friendly Fungicides for Plant Diseases Control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial Bacteria of Agricultural Importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Saeki, E.K.; Kobayashi, R.K.T.; Nakazato, G. Quorum Sensing System: Target to Control the Spread of Bacterial Infections. Microb. Pathog. 2020, 142, 104068. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic Screening and Expression Analysis of Psychrophilic Bacillus spp. Reveal Their Potential to Alleviate Cold Stress and Modulate Phytohormones in Wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef]
- Sekurova, O.N.; Schneider, O.; Zotchev, S.B. Novel Bioactive Natural Products from Bacteria via Bioprospecting, Genome Mining and Metabolic Engineering. Microb. Biotechnol. 2019, 12, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Farzand, A.; Moosa, A.; Zubair, M.; Rashid Khan, A.; Hanif, A.; Tahir, H.A.S.; Gao, X. Marker Assisted Detection and LC-MS Analysis of Antimicrobial Compounds in Different Bacillus Strains and Their Antifungal Effect on Sclerotinia sclerotiorum. Biol. Control 2019, 133, 91–102. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. 2017, 70, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; Zhang, H.; Pieterse, C.M.J.; Bolton, M.D.; De Jonge, R. Microbial Small Molecules-Weapons of Plant Subversion. Nat. Prod. Rep. 2018, 35, 410–433. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Huang, J.; Muhammad, M.; Deng, Z.; Gao, J. Genome Mining as a Biotechnological Tool for the Discovery of Novel Marine Natural Products. Crit. Rev. Biotechnol. 2020, 40, 571–589. [Google Scholar] [CrossRef]
- Mohammadi, P.; Tozlu, E.; Kotan, R.; Şenol Kotan, M. Potential of Some Bacteria for Biological Control of Postharvest Citrus Green Mould Caused by Penicillium digitatum. Plant Prot. Sci. 2017, 53, 134–143. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS Line with Gene Btcry1Ia Encoding Cry1Ia Toxin from Bacillus thuringiensis Promotes Integrated Wheat Defense against Pathogen Stagonospora nodorum Berk. and Greenbug Schizaphis graminum Rond. Biol. Control 2020, 144, 104242. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus thuringiensis Strains to Identify New Potential Biocontrol Agents against Sclerotinia sclerotiorum and Plutella xylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Yu, C.; Chen, H.; Zhu, L.; Song, Y.; Jiang, Q.; Zhang, Y.; Ali, Q.; Gu, Q.; Gao, X.; Borriss, R. Profiling of Antimicrobial Metabolites Synthesized by the Endophytic and Genetically Amenable Biocontrol Strain Bacillus velezensis DMW1. Microbiol. Spectr. 2023, 11, e00038-23. [Google Scholar] [CrossRef]
- Rajer, F.U.; Samma, M.K.; Ali, Q.; Rajar, W.A.; Wu, H.; Raza, W.; Xie, Y.; Tahir, H.A.S.; Gao, X. Bacillus spp. Mediated Growth Promotion of Rice Seedlings and Suppression of Bacterial Blight Disease under Greenhouse Conditions. Pathogens 2022, 11, 1251. [Google Scholar] [CrossRef]
- Liang, Z.; Ali, Q.; Wang, Y.; Mu, G.; Kan, X.; Ren, Y.; Manghwar, H.; Gu, Q.; Wu, H.; Gao, X. Toxicity of Bacillus thuringiensis Strains Derived from the Novel Crystal Protein Cry31Aa with High Nematicidal Activity against Rice Parasitic Nematode Aphelenchoides besseyi. Int. J. Mol. Sci. 2022, 23, 8189. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Qiao, J.; Wang, R.; Lu, J.; Wang, Z.; Li, P.; Zhang, L.; Ali, Q.; Khan, A.R.; Gao, X. The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize. Int. J. Mol. Sci. 2022, 23, 6431. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Zhang, X.; Zhang, M.; Gu, Y.; Ali, Q.; Mohamed, M.; Xu, J.; Shi, J.; Gao, X. Mycosubtilin Produced by Bacillus subtilis ATCC6633 Inhibits Growth and Mycotoxin Biosynthesis of Fusarium graminearum and Fusarium verticillioides. Toxins 2021, 13, 791. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Yu, C.; Wang, Y.; Sheng, T.; Zhao, X. High Killing Rate of Nematode and Promotion of Rice Growth by Synthetic Volatiles from Bacillus Strains Due to Enhanced Oxidative Stress Response. Physiol. Plant. 2023, 175, e13868. [Google Scholar] [CrossRef] [PubMed]
- Kakembo, D.; Lee, Y.H. Analysis of Traits for Biocontrol Performance of Pseudomonas parafulva JBCS1880 against Bacterial Pustule in Soybean Plants. Biol. Control 2019, 134, 72–81. [Google Scholar] [CrossRef]
- Patel, A.; Kumar, A.; Sheoran, N.; Kumar, M.; Sahu, K.P.; Ganeshan, P.; Ashajyothi, M.; Gopalakrishnan, S.; Gogoi, R. Antifungal and Defense Elicitor Activities of Pyrazines Identified in Endophytic Pseudomonas putida BP25 against Fungal Blast Incited by Magnaporthe oryzae in Rice. J. Plant Dis. Prot. 2021, 128, 261–272. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Wang, X.; Zheng, L.; Liu, H. Inhibitory e Ff Ects of Bacillus licheniformis BL06 on Phytophthora capsici in Pepper by Multiple Modes of Action. Biol. Control. 2020, 144, 104210. [Google Scholar] [CrossRef]
- Le, K.D.; Yu, N.H.; Park, A.R.; Park, D.J.; Kim, C.J.; Kim, J.C. Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases. Microorganisms 2022, 10, 791. [Google Scholar] [CrossRef]
- Hanawi, M.J. Tagetes erecta with Native Isolates of Paecilomyces lilacinus and Trichoderma hamatum in Controlling Root-Knot Nematode Meloidogyne javanica on Tomato. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 81–88. [Google Scholar]
- Kiriga, A.W.; Haukeland, S.; Kariuki, G.M.; Coyne, D.L.; Beek, N.V. Effect of Trichoderma spp. and Purpureocillium lilacinum on Meloidogyne javanica in Commercial Pineapple Production in Kenya. Biol. Control 2018, 119, 27–32. [Google Scholar] [CrossRef]
- Rivera-Mendez, W.; Obregón, M.; Moran-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma asperellum Biocontrol Activity and Induction of Systemic Defenses against Sclerotium cepivorum in Onion Plants under Tropical Climate Conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Siddiqui, M.A. Interactive Effect of Trichoderma virens and Meloidogyne incognita and Their Influence on Plant Growth Character and Nematode Multiplication on Abelmoschus esculentus (L.) Moench. Curr. Nematol. 2018, 29, 1–9. [Google Scholar]
- Bontempo, A.F.; Lopes, E.A.; Fernandes, R.H.; Freitas, L.G.D.E.; Dallemole-Giaretta, R. Dose-Response Effect of Pochonia chlamydosporia against Meloidogyne incognita on Carrot under Field Conditions. Rev. Caatinga 2017, 30, 258–262. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Guzmán-Ortiz, D.A.; Gómez-Lim, M.A.; Délano-Frier, J.P.; de-Folter, S.; Sánchez-García, P.; Peña-Cabriales, J.J. Potential Use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a Biological Control Agent against Anthracnose in Mango (Mangifera indica L.). Biol. Control 2013, 64, 37–44. [Google Scholar] [CrossRef]
- Geraldine, A.M.; Lopes, F.A.C.; Carvalho, D.D.C.; Barbosa, E.T.; Rodrigues, A.R.; Brandão, R.S.; Ulhoa, C.J.; Junior, M.L. Cell Wall-Degrading Enzymes and Parasitism of Sclerotia Are Key Factors on Field Biocontrol of White Mold by Trichoderma spp. Biol. Control 2013, 67, 308–316. [Google Scholar] [CrossRef]
- Jones, E.E.; Rabeendran, N.; Stewart, A. Biocontrol of Sclerotinia sclerotiorum Infection of Cabbage by Coniothyrium minitans and Trichoderma spp. Biocontrol Sci. Technol. 2014, 24, 1363–1382. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Safdar, A.; Huang, Z.; Rajer, F.U.; Gao, X. Effect of Volatile Compounds Produced by Ralstonia solanacearum on Plant Growth Promoting and Systemic Resistance Inducing Potential of Bacillus Volatiles. BMC Plant Biol. 2017, 17, 133. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Jiang, Q.; Wang, R.; Wang, Z.; Mu, G.; Khan, S.A.; Khan, A.R.; Manghwar, H.; Wu, H.; et al. Salt Tolerant Bacillus Strains Improve Plant Growth Traits and Regulation of Phytohormones in Wheat under Salinity Stress. Plants 2022, 11, 2769. [Google Scholar] [CrossRef]
- da Silva, L.R.; Inglis, M.C.V.; Moraes, M.C.B.; Magalhães, D.M.; Sifuentes, D.N.; Martins, I.; de Mello, S.C.M. Morphological and Protein Alterations in Sclerotinia sclerotiorum (Lib.) de Bary after Exposure to Volatile Organic Compounds of Trichoderma spp. Biol. Control 2020, 147, 104279. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, Y.; Yin, N.; Li, Y.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the Activity and Biological Control Efficacy of the Bacillus subtilis Strain Bs-1 against Meloidogyne incognita. Crop Prot. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Amma, D.K.B.N.S. Endophytic Bacterial Strains Induced Systemic Resistance in Agriculturally Important Crop Plants. In Microbial Endophytes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–105. [Google Scholar]
- Li, Y.; Héloir, M.-C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and Fengycin Contribute to the Protection of a Bacillus subtilis Strain against Grape Downy Mildew by Both Direct Effect and Defence Stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, H.; Chen, L.; Xie, S.; Zang, H.; Borriss, R.; Gao, X. Bacilysin from Bacillus amyloliquefaciens FZB42 Has Specific Bactericidal Activity against Harmful Algal Bloom Species. Appl. Environ. Microbiol. 2014, 80, 7512–7520. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete Genome Sequence of Bacillus velezensis QST713: A Biocontrol Agent That Protects Agaricus bisporus Crops against the Green Mould Disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S.; Leonetti, P. Bio-Control Agents Activate Plant Immune Response and Prime Susceptible Tomato against Root-Knot Nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef]
- Li, C.; Cheng, P.; Zheng, L.; Li, Y.; Chen, Y.; Wen, S.; Yu, G. Comparative Genomics Analysis of Two Banana Fusarium Wilt Biocontrol Endophytes Bacillus subtilis R31 and TR21 Provides Insights into Their Differences on Phytobeneficial Trait. Genomics 2021, 113, 900–909. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, T.; Shen, A.; Yang, X.; Yu, Y.; Gao, C.; Li, Z.; Cheng, Y.; Chen, J.; Guo, L. Biocontrol Potential of Bacillus subtilis IBFCBF-4 against Fusarium Wilt of Watermelon. J. Plant Pathol. 2020, 102, 433–441. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Zimmermann, S.D.; Marqués-gálvez, J.E. Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa? Front. Plant Sci. 2021, 12, 791033. [Google Scholar] [CrossRef]
- Alblooshi, A.A.; Purayil, G.P.; Saeed, E.E.; Ramadan, G.A.; Tariq, S.; Altaee, A.S.; El-Tarabily, K.A.; AbuQamar, S.F. Biocontrol Potential of Endophytic Actinobacteria against Fusarium solani, the Causal Agent of Sudden Decline Syndrome on Date Palm in the UAE. J. Fungi 2021, 8, 8. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as Biocontrol Agent against Pests: New Uses for a Mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Premjanu, N.; Jayanthy, C. Endophytic Fungi a Repository of Bioactive Compounds-a Review. Int. J. Institutional Pharm. Life Sci. 2012, 2, 135–162. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological Functions of Trichoderma spp. and Their Secondary Metabolites in the Rhizosphere: Interactions with Plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [PubMed]
- Risoli, S.; Cotrozzi, L.; Sarrocco, S.; Nuzzaci, M.; Pellegrini, E.; Vitti, A. Trichoderma-Induced Resistance to Botrytis cinerea in Solanum Species: A Meta-Analysis. Plants 2022, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Degani, O.; Dor, S. Trichoderma Biological Control to Protect Sensitive Maize Hybrids against Late Wilt Disease in the Field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef]
- Djonović, S.; Vittone, G.; Mendoza-Herrera, A.; Kenerley, C.M. Enhanced Biocontrol Activity of Trichoderma virens Transformants Constitutively Coexpressing β-1,3- and β-1,6-Glucanase Genes. Mol. Plant Pathol. 2007, 8, 469–480. [Google Scholar] [CrossRef]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA-Signaling Pathway and Ethylene Biosynthesis in Trichoderma harzianum Treated Tomato Plants after Infection of the Root-Knot Nematode Meloidogyne incognita. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological Control of Fungal Diseases by Trichoderma aggressivum f. europaeum and Its Compatibility with Fungicides. J. Fungi 2021, 7, 598. [Google Scholar]
- Yu, Z.; Wang, Z.; Zhang, Y.; Wang, Y.; Liu, Z. Biocontrol and Growth-Promoting Effect of Trichoderma asperellum TaspHu1 Isolate from Juglans mandshurica Rhizosphere Soil. Microbiol. Res. 2021, 242, 126596. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Hwang, B.S.; Baek, K.H. Bacillus velezensis: A Beneficial Biocontrol Agent or Facultative Phytopathogen for Sustainable Agriculture. Agronomy 2023, 13, 840. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Shaharoona, B.; Mahmood, T. Plant Growth Promoting Rhizobacteria and Sustainable Agriculture. In Microbial Strategies for Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–160. [Google Scholar]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Niu, Y.; Huo, R.; Gao, X. Bacillus Volatiles Adversely Affect the Physiology and Ultra-Structure of Ralstonia solanacearum and Induce Systemic Resistance in Tobacco against Bacterial Wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G. Biocontrol Potential and Plant-Growth-Promoting Effects of Bacillus amyloliquefaciens MBI 600 against Fusarium oxysporum f. sp. radicis-lycopersici on Tomato. Acta Hortic. 2018, 1207, 139–145. [Google Scholar] [CrossRef]
- Srivastava, S.S.; Bist, V.; Srivastava, S.S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Garzón, J.F.G.; Weber, T. Recent Advances in Re-Engineering Modular PKS and NRPS Assembly Lines. Biotechnol. Bioprocess Eng. 2020, 25, 886–894. [Google Scholar] [CrossRef]
- Prasanth, M.; Ramesh, N.; Gothandam, K.M.; Sivamangala, K.; Shanthini, T. Pseudomonas syringae: An Overview and Its Future as a “Rain Making Bacteria”. Int. Res. J. Biol. Sci. 2015, 4, 70–77. [Google Scholar]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest Biology and Technology Postharvest Biocontrol Ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on Stone Fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus amyloliquefaciens S76-3 from Wheat Spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef]
- Issazadeh, K.; Rad, S.K.; Zarrabi, S.; Rahimibashar, M.R. Antagonism of Bacillus Species against Xanthomonas campestris pv. campestris and Pectobacterium carotovorum subsp. carotovorum. Afr. J. Microbiol. Res. 2012, 6, 1615–1620. [Google Scholar] [CrossRef]
- Gu, Y.; Zheng, R.; Sun, C.; Wu, S. Isolation, Identification and Characterization of Two Kinds of Deep-Sea Bacterial Lipopeptides Against Foodborne Pathogens. Front. Microbiol. 2022, 13, 792755. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, J.P.; Zhu, Y.J.; Liu, B.; Yang, W.J.; Ruan, C.Q. Antibacterial Activity against Ralstonia solanacearum of the Lipopeptides Secreted from the Bacillus amyloliquefaciens Strain FJAT-2349. J. Appl. Microbiol. 2019, 126, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.A.S.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yin, X.; Wang, Q.; Peng, Y.; Liu, P.; Agricultural, S.; Province, S.; Shi, J. Antagonistic Activities of Volatiles Produced by Two Bacillus Strains Against Monilinia fructicola in Peach Fruit. J. Sci. Food Agric. 2018, 98, 5756–5763. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; El-Mageed, T.A.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major Secondary Metabolites Produced by Two Commercial Trichoderma Strains Active against Different Phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef]
- Shanthiyaa, V.; Saravanakumar, D.; Rajendran, L.; Karthikeyan, G.; Prabakar, K.; Raguchander, T. Use of Chaetomium globosum for Biocontrol of Potato Late Blight Disease. Crop Prot. 2013, 52, 33–38. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as Biological Control Agent: Scope and Prospects to Improve Efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Mosaddeghi, M.R.; Hosseini, F.; Hajabbasi, M.A.; Sabzalian, M.R.; Sepehri, M. Epichloë spp. and Serendipita indica Endophytic Fungi: Functions in Plant-Soil Relations. Adv. Agron. 2021, 165, 59–113. [Google Scholar]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Babalola, O.O. Metagenomics: A Tool for Exploring Key Microbiome With the Potentials for Improving Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 1–15. [Google Scholar] [CrossRef]
- Umer, M.; Mubeen, M.; Iftikhar, Y.; Shad, M.A.; Usman, H.M.; Sohail, M.A.; Atiq, M.N.; Abbas, A.; Ateeq, M. Role of Rhizobacteria on Plants Growth and Biological Control of Plant Diseases: A Review. Plant Prot. 2021, 5, 59–73. [Google Scholar]
- Oa, S.B.C.W.; Rudrappa, T.; Czymmek, K.J.; Pare, P.W. Root-Secreted Malic Acid Recruits Beneficial. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to Plant–Microbe Interactions Provide Opportunities to Improve Resistance Breeding against Root Diseases in Grain Legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef]
- Sébastien, M.; Margarita, M.; Haissam, J.M. Biological Control in the Microbiome Era: Challenges and Opportunities. Biol. Control 2015, 89, 98–108. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial Interactions and Biocontrol in the Rhizosphere. J. Exp. Bot. 2001, 52, 487. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Timper, P. Conserving and Enhancing Biological Control of Nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and Plants–With Special Reference to Induced Systemic Resistance (ISR). Microbiol. Res. 2008, 164, 493–513. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the Efficacy of Biological Control against Plant Diseases Likely to Be More Durable than That of Chemical Pesticides. Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Akutse, K.S.; Subramanian, S.; Maniania, N.K.; Dubois, T.; Ekesi, S. Biopesticide Research and Product Development in Africa for Sustainable Agriculture and Food Security–Experiences From the International Centre of Insect Physiology and Ecology (Icipe). Front. Sustain. Food Syst. 2020, 4, 563016. [Google Scholar] [CrossRef]
- Ezrari, S.; Mhidra, O.; Radouane, N.; Tahiri, A.; Polizzi, G.; Lazraq, A.; Lahlali, R. Potential Role of Rhizobacteria Isolated from Citrus Rhizosphere for Biological Control of Citrus Dry Root Rot. Plants 2021, 10, 872. [Google Scholar] [CrossRef]
- Nicot, P.C.; Alabouvette, C.; Bardin, M.; Blum, B.; Köhl, J.; Ruocco, M. Review of Factors Influencing the Success or Failure of Biocontrol: Technical, Industrial and Socio-Economic Perspectives. IOBC-WPRS Bull. 2012, 78, 95–98. [Google Scholar]
- Sehrawat, A.; Sindhu, S.S. Potential of Biocontrol Agents in Plant Disease Control for Improving Food Safety. Def. Life Sci. J. 2019, 4, 220–225. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The Status of Biological Control and Recommendations for Improving Uptake for the Future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Mark, G.L.; Morrissey, J.P.; Higgins, P.; O’Gara, F. Molecular-Based Strategies to Exploit Pseudomonas Biocontrol Strains for Environmental Biotechnology Applications. FEMS Microbiol. Ecol. 2006, 56, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When Is It Biological Control? A Framework of Definitions, Mechanisms, and Classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Farrar, K.; Bryant, D.; Cope-Selby, N. Understanding and Engineering Beneficial Plant-Microbe Interactions: Plant Growth Promotion in Energy Crops. Plant Biotechnol. J. 2014, 12, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Gullino, M.L. State of the Art and Future Prospects of the Biological Control of Postharvest Fruit Diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ayaz, M.; Mu, G.; Hussain, A.; Yuanyuan, Q.; Yu, C.; Xu, Y.; Manghwar, H.; Gu, Q.; Wu, H.; et al. Revealing Plant Growth-Promoting Mechanisms of Bacillus Strains in Elevating Rice Growth and Its Interaction with Salt Stress. Front. Plant Sci. 2022, 13, 994902. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.; Latz, M.A.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of Fungal Endophytes with Biocontrol Potential against Fusarium Head Blight in Wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Marina, T.A.-E.; Maythsulene, I.S.O.; Valacia, L.S.-L.; Marta, C.C.F.; Amadou, H.B.; Edemilson, C.C.; Marcio, V. de C.B.C. Shelf Life Enhancement of Plant Growth Promoting Rhizobacteria Using a Simple Formulation Screening Method. Afr. J. Microbiol. Res. 2018, 12, 115–126. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the Framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2013, 70, 2–5. [Google Scholar] [CrossRef]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Sundh, I.; Eilenberg, J. Why Has the Authorization of Microbial Biological Control Agents Been Slower in the EU than in Comparable Jurisdictions. Pest Manag. Sci. 2020, 77, 2170–2178. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Hidayah, B.N. Biological Control Potential of Trichoderma Species and Bacterial Antagonists against Sclerotinia sclerotiorum on Canola in Western Australia. Int. J. Agric. Biol. 2022, 27, 215–227. [Google Scholar] [CrossRef]
- Waage, J.K.; Greathead, D.J. Biological Control: Challenges and Opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988, 318, 111–128. [Google Scholar] [CrossRef]
- Brodeur, J.; Abram, P.K.; Heimpel, G.E.; Messing, R.H. Trends in Biological Control: Public Interest, International Networking and Research Direction. BioControl 2018, 63, 11–26. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Sundh, I.; Goettel, M.S. Regulating Biocontrol Agents: A Historical Perspective and a Critical Examination Comparing Microbial and Macrobial Agents. BioControl 2012, 58, 575–593. [Google Scholar] [CrossRef]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial Biopesticides for Insect Pest Management in India: Current Status and Future Prospects. J. Invertebr. Pathol. 2018, 165, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and Implementation of Biocontrol 1. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Wilson, M.J.; Jackson, T.A. Progress in the Commercialisation of Bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic Gene Clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Nogueira, T.; Botelho, A. Metagenomics and Other Omics Approaches to Bacterial Communities and Antimicrobial Resistance Assessment in Aquacultures. Antibiotics 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Ibrahim, D.S.S.; Belabess, Z.; Roni, M.Z.K.; Radouane, N.; Vicente, C.S.L.; Menendez, E.; Mokrini, F.; Barka, E.A.; de Melo e Mota, M.G.; et al. High-Throughput Molecular Technologies for Unraveling the Mystery of Soil Microbial Community: Challenges and Future Prospects. Heliyon 2021, 7, e08142. [Google Scholar] [CrossRef] [PubMed]
- Demoz, B.T.; Korsten, L. Bacillus subtilis Attachment, Colonization, and Survival on Avocado Flowers and Its Mode of Action on Stem-End Rot Pathogens. Biol. Control 2006, 37, 68–74. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Rocha-Granados, M.d.C.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Raizada, M.N. The Diversity of Anti-Microbial Secondary Metabolites Produced by Fungal Endophytes: An Interdisciplinary Perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wei, H.; Liu, X.; Wang, Y.; Zhang, L.; Tang, W. Improving Biocontrol Activity of Pseudomonas fluorescens through Chromosomal Integration of 2, 4-Diacetylphloroglucinol Biosynthesis Genes. Chin. Sci. Bull. 2005, 50, 775–781. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Shao, J.; Li, S.; Zhang, N.; Cui, X.; Zhou, X.; Zhang, G.; Shen, Q.; Zhang, R. Analysis and Cloning of the Synthetic Pathway of the Phytohormone Indole-3-Acetic Acid in the Plant-Beneficial Bacillus amyloliquefaciens SQR9. Microb. Cell Fact. 2015, 14, 130. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, A.; Tan, H.; Cao, L.; Zhang, R. Engineering Banana Endosphere Microbiome to Improve Fusarium Wilt Resistance in Banana. Microbiome 2019, 7, 74. [Google Scholar] [CrossRef]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome Engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Barrangou Jan-Peter, R. van P. Exploiting CRISPR-Cas Immune Systems for Genome Editing in Bacteria. Curr. Opin. Biotechnol. 2015, 37, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Feklistova, I.N.; Maksimova, N.P. Obtaining Pseudomonas aurantiaca Strains Capable of Overproduction of Phenazine Antibiotics. Microbiology 2008, 77, 176–180. [Google Scholar] [CrossRef]
- Larena, I.; Espeso, E.A.; Veloso, J. Editorial: Impact of Novel Omic Technologies on Biological Control against Plant Pathogens. Front. Microbiol. 2023, 14, 1162422. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariëns, S.; Babendreier, D.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.A.; Centurión, A.; et al. Next-Generation Biological Control: The Need for Integrating Genetics and Genomics. Biol. Rev. 2020, 95, 1838–1854. [Google Scholar] [CrossRef]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” Tools for Better Understanding the Plant–Endophyte Interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gold, K.M.; Jiménez-Gasco, M.d.M.; Filgueiras, C.C.; Willett, D.S. A Multi-Omics Approach to Solving Problems in Plant Disease Ecology. PLoS ONE 2020, 15, e0237975. [Google Scholar] [CrossRef]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome Profiling of the Endophyte Burkholderia phytofirmans Psjn Indicates Sensing of the Plant Environment and Drought Stress. MBio 2015, 6, e00621-15. [Google Scholar] [CrossRef]
- Manjula, A.; Narsimha, G. XCYPF: A Flexible and Extensible Framework for Agricultural Crop Yield Prediction. In Proceedings of the 2015 IEEE 9th International Conference on Intelligent Systems and Control (ISCO), Coimbatore, India, 9–10 January 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–5. [Google Scholar]
- Bhattacharyya, P.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2011, 28, 1327–1350. [Google Scholar] [CrossRef]
- Köberl, M.; Schmidt, R.; Ramadan, E.M.; Bauer, R.; Berg, G. The Microbiome of Medicinal Plants: Diversity and Importance for Plant Growth, Quality and Health. Front. Microbiol. 2013, 4, 400. [Google Scholar] [CrossRef]
- Zachow, C.; Müller, H.; Tilcher, R.; Berg, G. Differences between the Rhizosphere Microbiome of Beta vulgaris ssp. maritima-Ancestor of All Beet Crops-and Modern Sugar Beets. Front. Microbiol. 2014, 5, 415. [Google Scholar] [CrossRef]
- Müller, H.; Berg, C.; Landa, B.B.; Auerbach, A.; Moissl-Eichinger, C.; Berg, G. Plant Genotype-Specific Archaeal and Bacterial Endophytes but Similar Bacillus Antagonists Colonize Mediterranean Olive Trees. Front. Microbiol. 2015, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Ramadan, E.M.; Adam, M.; Cardinale, M.; Hallmann, J.; Heuer, H.; Smalla, K.; Berg, G. Bacillus and Streptomyces Were Selected as Broad-Spectrum Antagonists against Soilborne Pathogens from Arid Areas in Egypt. FEMS Microbiol. Lett. 2013, 342, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, S.; Kumar, P. Rhizosphere Microbiome: Revisiting the Synergy of Plant-Microbe Interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Berg, G.; Zachow, C.; Müller, H.; Philipps, J.; Tilcher, R. Next-Generation Bio-Products Sowing the Seeds of Success for Sustainable Agriculture. Agronomy 2013, 3, 648–656. [Google Scholar] [CrossRef]
- Marco, S.; Loredana, M.; Riccardo, V.; Raffaella, B.; Walter, C.; Luca, N. Microbe-Assisted Crop Improvement: A Sustainable Weapon to Restore Holobiont Functionality and Resilience. Hortic. Res. 2022, 9, uhac160. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; Patz, S.; Ruppel, S.; Dietel, K.; Faetke, S.; Junge, H.; Becker, M. Successful Formulation and Application of Plant Growth-Promoting Kosakonia radicincitans in Maize Cultivation. BioMed Res. Int. 2018, 2018, 6439481. [Google Scholar] [CrossRef]
- Massalha, H.; Korenblum, E.; Malitsky, S.; Shapiro, O.H.; Aharoni, A. Live Imaging of Root-Bacteria Interactions in a Microfluidics Setup. Proc. Natl. Acad. Sci. USA 2017, 114, 4549–4554. [Google Scholar] [CrossRef]



| Plant Species | Biocontrol Agents | Pathogens | Mode of Action | Ref. |
|---|---|---|---|---|
| Bacterial strains | ||||
| Citrus fruit | Bacillus megaterium | Blue mold | In vitro antagonistic activity against post-harvest disease | [16] |
| Wheat | Bacillus subtilis 26DCryChS | Stagonospora nodorum Berk | Antimicrobial metabolites (surfactants showed antifungal activity against S. nodorum disease) | [17] |
| Brassica campestris L | Bacillus thuringiensis | Sclerotinia sclerotiorum | Suppressing S. sclerotiorum growth by inducing systemic resistance | [18] |
| Cotton/black root rot | Paenibacillus alvei K-165 | Thielaviopsis basicola | K-165 inhibited T. basicola growth invitro through antibiosis and significantly reduced root discoloration and hypocotyl lesions on cotton seedlings | [6] |
| Tomato and Soybean | Bacillus velezensis DMW1 | Phytophthora sojae and Ralstonia solanacearum | Antimicrobial metabolites (fengycin, iturin, and bacillomycin) demonstrated antagonistic activity in vitro and in pot experiments | [19] |
| Rice | Bacillus atrophaeus FA12 and B. cabrialesii FA26 | Xanthomonas oryzae pv. oryzae (Xoo) | In vitro, antagonistic activity against various fungal pathogens significantly reduced Xoo lesions in greenhouse conditions | [20] |
| Rice | Bacillus thuringiensis GBAC46 | Aphelenchoides besseyi | In vitro antagonistic activity through various proteins (Cry31Aa, Cry73Aa, and Cry40ORF) and in greenhouse conditions | [21] |
| Maize | Pseudomonas protegens Pf-5 | Pantoea ananatis DZ-12 | Antimicrobial pyoluteorin showed strong antagonistic activity against P. ananatis in vitro and in vivo | [22] |
| Wheat and Maize | Bacillus Subtilis ATCC6633 | Fusarium graminearum and Fusarium verticillioides | Antimicrobial mycosubtilin showed a strong antagonistic activity against F. graminearum and F. verticillioides in vitro and in vivo | [23] |
| Tomato | Bacillus atrophaeus GBSC56 | Meloidogyne incognita | Antimicrobial VOCs showed nematicidal activity and also produced ROS in nematodes | [2] |
| Rice | Bacillus spp. GBSC56, SYST2, and FZB42 | Aphelenchoides besseyi | Antimicrobial VOCs of Bacillus spp. showed the strongest nematicidal activity and accumulated ROS as well as promoted rice growth | [24] |
| Soybean and Rice | Pseudomonas parafulva JBCS1880 | Xanthomonas axonopodis pv. glycines, and Burkholderia glumae | Strong antagonism and antibacterial activity against Xanthomonas axonopodis pv. glycines and Burkholderia glumae | [25] |
| Rice | Pseudomonas putida BP25 | Magnaporthe oryzae | BP25 showed strong biocontrol activity against blasts caused by M. oryzae | [26] |
| Pepper | Bacillus licheniformis BL06 | Phytophthora capsici | BL06 effectively reduced pepper Phytophthora blight severity in vitro and pot experiments | [27] |
| Wheat | Bacillus atrophaeus strain TS1 | Fusarium graminearum | TS1 was found as a potential biocontrol agent to inhibit F. graminearum under low temperatures | [5] |
| Tomato | Bacillus amyloliquefaciens FZB42 | Sclerotinia sclerotiorum | Antimicrobial potential (fengycin-induced systemic resistance in tomatoes against S. sclerotiorum) | [1] |
| Rape Seed and Tabaco | Bacillus amyloliquefaciens EZ1509 | Sclerotinia sclerotiorum | Bacillus strain EZ1509 showed a strong antifungal activity against S. sclerotiorum and also led to the development of new biopesticides | [12] |
| Tomato | Streptomyces sp. AN090126 | Ralstonia solanacearum and Xanthomonas euvesicatoria | Streptomyces sp. AN090126 can combine with antibiotics effectively control different bacterial plant diseases | [28] |
| Fungal strains | ||||
| Tomato | Paecilomyces lilacinus | Meloidogyne javanica | P. lilacinum is used as a biocontrol agent to control M. incognita and as a better alternative against chemical nematicides | [29] |
| Pineapple | Purpureocillium lilacinum | Meloidogyne javanica | The application of P. lilacinum significantly reduced nematode egg and egg mass production, reducing root galling damage in pineapple | [30] |
| Onion | Trichoderma asperellum | Sclerotium cepivorum | T. asperellum BCC1 exerts efficient biocontrol against S. cepivorum and activates onion systemic defenses against S. cepivorum under greenhouse conditions | [31] |
| Okra | Trichoderma virens | Meloidogyne incognita | T. virens observed a reduction in second-stage juveniles’ hatching periods tested in vitro | [32] |
| Carrot | Pochonia chlamydosporia | Meloidogyne incognita | P. chlamydosporia reduced nematode galls and also decreased juvenile 2 nematodes in vitro and pot experiment methods | [33] |
| Mango | Trichoderma asperellum T8a | Colletrotrichum gloeosporiodes | T. asperellum T8a plays a role in biological control against C. gloeosporioides and controlling anthracnose disease in mangoes | [34] |
| Beans | Trichoderma asperellum | Sclerotinia sclerotiorum | T. asperellum the reduced disease severity index and antagonistic activity against S. sclerotiorum in field trials of beans | [35] |
| Cabbage | Trichoderma hamatum | Sclerotinia sclerotiorum | T. hamatum LU593 reduced apothecial production, decreased disease severity index, and could potentially control S. sclerotiorum disease in cabbage | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. https://doi.org/10.3390/molecules28186735
Ayaz M, Li C-H, Ali Q, Zhao W, Chi Y-K, Shafiq M, Ali F, Yu X-Y, Yu Q, Zhao J-T, et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules. 2023; 28(18):6735. https://doi.org/10.3390/molecules28186735
Chicago/Turabian StyleAyaz, Muhammad, Cai-Hong Li, Qurban Ali, Wei Zhao, Yuan-Kai Chi, Muhammad Shafiq, Farman Ali, Xi-Yue Yu, Qing Yu, Jing-Tian Zhao, and et al. 2023. "Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives" Molecules 28, no. 18: 6735. https://doi.org/10.3390/molecules28186735
APA StyleAyaz, M., Li, C. -H., Ali, Q., Zhao, W., Chi, Y. -K., Shafiq, M., Ali, F., Yu, X. -Y., Yu, Q., Zhao, J. -T., Yu, J. -W., Qi, R. -D., & Huang, W. -K. (2023). Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules, 28(18), 6735. https://doi.org/10.3390/molecules28186735