Cinnamomum sp. and Pelargonium odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Content, Total Flavonoid Content, and Total Antioxidant Capacity
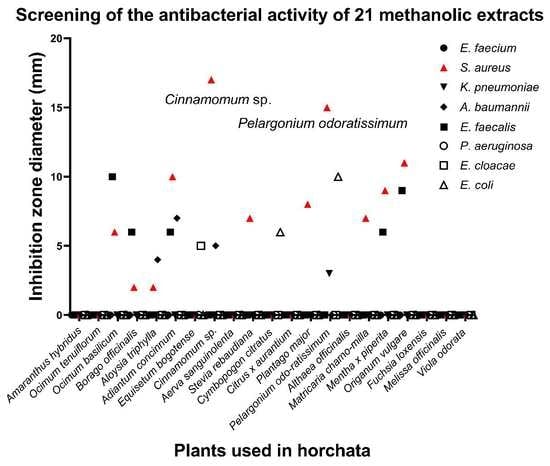
2.2. Antibacterial Activity of Crude Extracts
2.3. Biofilm Inhibition and Eradication Assays
2.4. Identification of Chemical Compounds in Cinnamomum sp. and Pelargonium odoratissimum (L.) L’Hér Hydroalcoholic Extracts
| Positive Ionisation | ||||||
|---|---|---|---|---|---|---|
| ID | RT (min) | UV | Parent Mass (m/z) | Main MS/MS Products (m/z) | Tentative Identification | References |
| 1 | 1.16 | 250, 266 | 203 ([M+Na]+) | 185 (100), 113 (90), 143 (50), 172 (20) | Fructopyranose | [29] |
| 1.21 | 250, 266 | 219 ([M+K]+) | 201 (100), 89 (45), 210 (10), 72 (10) | Fructose | [30] | |
| 1.27 | 250, 266 | 381 ([2-hexose+O+Na]+) | 363 (100), 219 (30), 297 (25), 201 (15), 345 (15) | Disaccharide | [23] | |
| 1.27 | 250, 266 | 266 ([M+H]+) | 248 (100), 230 (5) | UI | ||
| 1.27 | 250, 266 | 180 ([M+H]+) | 121 (100), 165 (40), 60 (30) | Candicine | [22] | |
| 2 | 3.06 | 222, 274 | 180 ([M+H]+) | 121 (100), 165 (40), 60 (30) | Candicine isomer | [22], see comments |
| 3 | 6.42 | 214, 278 | 223 ([M+H]+) | 207 (100), 225 (15) | 4-(2,3-Dihydro-1,4-benzodioxin-6-yl)butanoic acid | 86.8% |
| 4 | 6.47 | 214, 278 | 208 ([M+H]+) | 131 (100), 149 (50), 103 (10) | UI | |
| 5 | 9.87 | 214, 278 | 1153 ([M+H]+) | 1001 (100), 863 (50), 985 (35) | A type (Epi)catechin tetramer (EC-EC-EC-A-EC-EC) | [24], see comments |
| 6 | 11.56 | 214, 278 | 314 ([M+H]+) | 297 (100), 298 (98), 269 (60), 175 (10), 237 (5) | Lotusine | [27], see comments |
| 11.69 | 218, 278 | 1153 ([M+H]+) | 865 (100), 1001 (90), 983 (45), 985 (40) | A Type procyanidin pentamer | [24], see comments | |
| 11.83 | 218, 278 | 579 ([M+H]+) | 427 (100), 409 (80), 291 (70), 301 (25), 247 (20), 561 (5) | A type of proanthocyanidin B | [24,25], see comments | |
| 12.24 | 214, 278 | 286 ([M+H]+) | 269 (100), 175 (10), 107 (5), 237 (5) | Coclaurine | [31], see comments | |
| 7 | 12.43 | 230, 278 | 300 ([M+H]+) | 269 (100), 175 (15), 107 (5), 237 (5) | N-methylcoclaurine | [31], see comments |
| 12.43 | 230, 278 | 291 ([M+H]+) | 123 (100), 139 (85), 165 (45), 273 (35), 151 (30) | Epicatechin | 96.5% | |
| 12.83 | 218, 278 | 1153 ([M+H]+) | 865 (100), 1001 (90), 983 (45), 985 (40) | A type of procyanidin pentamer, isomer | [24], see comments | |
| 8 | 12.45–12.97 | 230, 278 | 865 ([M+H]+) | 533 (100), 713 (60), 828 (35), 695 (30) | A type procyanidin trimer (EC-A-EC-EC) | [24,25], see comments |
| 13.00 | 218, 278 | 314 ([M+H]+) | 283 (100), 269 (40), 299 (25), 107 (20) | 4′-Methyl-N-methylcoclaurine | [27], see comments | |
| 9 | 13.38 | 214, 278 | 330 ([M+H]+) | 192 (100), 299 (10) | Reticuline | [32,33] |
| 15.04 | 214, 278 | 579 ([M+H]+) | 409 (100), 427 (75), 561 (50), 291 (50), 453 (50) | A type of procyanidin B isomer | see comments | |
| 10 | 16.88 | 214, 278 | 149 ([M+H]+) | 149 (100) | 2-Hydroxycinnamaldehyde | [28], see comments |
| 11 | 19.24 | 214, 278 | 693 ([M+H]+) | 541 (100), 523 (80), 389 (10) | UI | |
| 12 | 19.45 | 214, 274 | 149 ([M+H]+) | 149 (100) | Cinnamic acid | [28], see comments |
| 13 | 19.63 | 214, 278 | 423 ([M+H]+) | 405 (100), 387 (59), 271 (50) | Mangiferin | [34] |
| 14 | 20.14 | 214, 278 | 237 ([M+H]+) | 219 (100), 201 (60), 191 (60), 180 (40) | Prehelminthosporol | [35] |
| 15 | 22.70 | 214, 286, 388 | 195 ([M+H]+) | 163 (100), 154 (10), 167 (8), 180 (8), 135 (5) | Methoxycinnamaldehyde derivate | [28], see comments |
| 16 | 22.89 | 234, 290, 338 | 163 ([M+H]+) | 163 (100) | Methoxycinnamaldehyde (see notes) | [28], see comments |
| Negative ionisation | ||||||
| 1 | 1.25 | 230,278 | 191 ([M-H]−) | 111 (100), 173 (50), 85(25), 127 (20) | Quinic acid | 87.8% |
| 2 | 1.32 | 242, 278 | 195 ([M-H]−) | 129 (100), 177 (80), 159 (40), 141 (5), 111 (5) | Galactonic acid | 82.0% |
| 3 | 13.50 | 226, 286 | 206 ([M-H]−) | 164 (100), 147 (15) | N-acetyl-L-Phenylalanine | 97.9% |
| Positive Ionisation | ||||||
|---|---|---|---|---|---|---|
| ID | RT (min) | UV | Parent Mass (m/z) | Main MS/MS Products (m/z) | Tentative Identification | References |
| 1 | 1.06–1.55 | 250, 290 | 175 ([M+H]+) | 157 (100), 175 (50), 130 (60), 116 (45), 60 (35) | L-(+) arginine | 96.1% |
| 219 ([M+H]+) | 201 (100), 159 (25), 173 (20), 90 (10) | 5-Acetyl-2-(1-hydroxy-1-methylethyl)benzofuran | [37] | |||
| 222 | 182 ([M+H]+) | 165 (100), 136 (25) | L-tyrosine | 87.7% | ||
| 293 ([M+H]+) | 275 (100), 233 (40), 209 (20), 203 (15), 118 (10) | UI | see comments | |||
| 2 | 1.62 | 214, 270 | 371 ([M+H]+) | 283 (100), 353 (70) | UI | |
| 2.50 | 214, 266 | 166 ([M+H]+) | 166 (100), 120 (30) | L-phenylalanine | 93.9% | |
| 3 | 4.94 | 214, 270 | 611 ([M+H]+) | 443 (100), 425 (15),485 (5) | Polymer of gallocatechin | [36], see comments |
| 7.39 | 307 ([M+H]+) | 139 (100), 151 (80), 289 (80) | Epigallocatechin | 93.7% | ||
| 4 | 8.49 | 218, 278 | 205 ([M+H]+) | 188 (100), 159 (5) | L-Tryptophan | 95.9% |
| 5 | 10.28 | 218, 278 | 611 ([M+H]+) | 425 (100), 443 (80), 317 (35), 287 (30), 467 (30), 485 (30), 593 (30) | Polymer of gallocatechin | [36], see comments |
| 6 | 11.57 | 218, 278 | 389 ([M+H]+) | 209 (100), 227 (35), 191 (20), 371 (10), 173 (10) | 4-[4-Hydroxy-2,6,6-trimethyl-3-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxycyclohexen-1-yl]butan-2-one | 81.6% |
| 7 | 11.89 | 238, 282 | 690 ([M+H]+) | 316 (100) 316->298 (100), 209 (30),227 (25),177 (5) | UI | |
| 8 | 12.53 | 222, 274 | 783 ([M+H]+) | 303 (100), 277 (40), 337 (30), 463 (20),765 (10) | UI | |
| 9 | 12.69 | 230, 278 | 459 ([M+H]+) | 289 (100), 151 (20), 139 (20) | Epigallocatechin gallate | 95.0% |
| 10 | 13.80 | 218, 266, 350 | 449 ([M+H]+) | 431 (100), 383 (60), 353 (25), 329 (20) | Luteolin-8-glucoside | 95.2% |
| 11 | 13.85 | 226, 270, 350 | 449 ([M+H]+) | 431 (100), 383 (60), 353 (25), 329 (20) | Luteolin glucoside isomer | see comments |
| 12 | 13.87 | 218, 266, 346 | 348 ([M+H]+) | 169 (100), 331 (20), 123 (20), 313 (15), 151 (10) | UI | see comments |
| 14.62 | 222, 266, 342 | 619 ([M-H2O+H]+) | 449 (100), 237 (70), 261 (20), 279 (10), 431 (5) | 1,2,6-tri-O-galloylglucose | 88.8% | |
| 13 | 14.78 | 222, 266, 342 | 376 ([M+H]+) | 197 (100), 179 (20), 358 (5) | UI | |
| 14.91 | 611 ([M+H]+) | 303 (100), 465 (30) | Rutin | see comments | ||
| 14 | 15.00 | 222, 266, 342 | 433 ([M+H]+) | 415 (100), 367 (50), 271 (40), 397 (25), 337 (20), 313 (20), 379 (15) | Vitexin | 92.8% |
| 15.00 | 318, 266, 346 | 435 ([M+H]+) | 417 (100), 399 (25), 369 (20), 315 (15), 339 (10) | Naringenin-7-O-glucoside | 84.5%, see comments | |
| 15.24 | 222, 266, 342 | 465 ([M+H]+) | 303 (100), 447 (10) | Quercetin hexoside | see comments | |
| 15 | 15.38 | 218, 278 | 334 ([M+H]+) | 299 (100), 317 (25), 177 (5) | UI | |
| 16 | 15.93 | 214, 278 | 595 ([M+H]+) | 287 (100), 449 (30), 576 (5) 449-> 287 (100), 431 (90), 383 (45) | Kaempferol 3-neohesperidoside | 92.4%, see comments |
| 17 | 16.63 | 214, 278 | 449 ([M+H]+) | 287 (100), 431 (90), 383 (50) | Trifolin | 96.1%, see comments |
| 18 | 17.71 | 214, 286 | 481 ([M+H]+) | 303 (100), 285 (90), 301 (75), 463 (20), 319 (20) | Myricetin 3-galactoside | see comments |
| 19 | 18.61 | 214, 282 | 481 ([M+H]+) | 301 (100), 319 (50), 283 (45), 463 (20) | UI | |
| 20 | 19.50 | 214, 274 | 303 ([M+H]+) | 257 (100), 285 (35), 165 (5), 229 (5) | Quercetin | see comments |
| Negative ionisation | ||||||
| 21 | 1.06–1.55 | 250, 290, 314 | 169 ([M-H]−) | 169 (100), 125 (20) | Gallic acid | 87.1% |
| 331 ([M-H]−) | 169 (100), 271 (80), 211 (40), 125 (10) | Galloylglucose | [38] | |||
| 22 | 12.71 | 218, 278 | 951 ([M-H]−) | 907 (100), 934 (80) | Geraniin | Refs. [39,40,41], see comments |
| 23 | 14.07 | 214, 270, 346 | 447 ([M-H]−) | 327 (100), 357 (75), 429 (25) | 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-8-[(3R,4R,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | 84.0% |
| 24 | 14.79 | 222, 278, 338 | 951 ([M-H]−) | 934 (100), 933 (70) | Granatin B | Refs. [39,40,41], see comments |
| 25 | 15.15 | 230, 270, 338 | 301 ([M-H]−) | 257 (100), 185 (50) | Ellagic acid | [42] |
| 26 | 15.34 | 222, 270, 338 | 433 ([M-H]−) | 313 (100), 387 (30), 301 (25), 343 (10) | Hemiphloin | 75.4% |
| 27 | 17.50 | 214,274 | 429 ([M-H]−) | 249 (100), 267 (30) | Formononetin-7-O-glucoside | [43] |
3. Materials and Methods
3.1. Plant Materials and Extract Preparation
3.2. Total Phenolic Content, Total Flavonoid Content, and Total Antioxidant Capacity Assays
3.3. Bacterial Strains, Media, and Growth Conditions
3.4. Screening of Antibacterial Activity by the Well Diffusion Method
3.5. Antibacterial Activity by Microdilution Assay
3.6. Biofilm Inhibition Assay
3.7. Biofilm Eradication Assay
3.8. HPLC-DAD-MS/MS Analysis for Polyphenolic Composition
3.9. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Cowan, M.M. Plant Products as Antimicrobial Agents. Cosmet. Drug Microbiol. 2016, 12, 205–231. [Google Scholar] [CrossRef]
- Wright, G.D. Opportunities for Natural Products in 21st Century Antibiotic Discovery. Nat. Prod. Rep. 2017, 34, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Deng, Z. An Aurora of Natural Products-Based Drug Discovery Is Coming. Synth. Syst. Biotechnol. 2020, 5, 92–96. [Google Scholar] [CrossRef]
- Shrinet, K.; Singh, R.K.; Chaurasia, A.K.; Tripathi, A.; Kumar, A. Bioactive Compounds and Their Future Therapeutic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128206553. [Google Scholar]
- Keita, K.; Darkoh, C.; Okafor, F. Secondary Plant Metabolites as Potent Drug Candidates against Antimicrobial-Resistant Pathogens. SN Appl. Sci. 2022, 4, 209. [Google Scholar] [CrossRef]
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Stankov-Jovanović, V.; Mitić, V.; Jović, J.; Čomić, L.; Kocić, B.; Bernstein, N. Antibacterial and Antioxidant Activity of Traditional Medicinal Plants from the Balkan Peninsula. NJAS Wageningen J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G.; Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural Compounds and Extracts as Novel Antimicrobial Agents Natural Compounds and Extracts as Novel Antimicrobial Agents. Expert Opin. Ther. Pat. 2020, 30, 949–962. [Google Scholar] [CrossRef]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial Activity of Medicinal Plants against ESKAPE: An Update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef]
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Ekin Yalcinkaya, I.; Capanoglu, E. Phytochemicals of Herbs and Spices: Health versus Toxicological Effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef]
- Rios, M.; Tinitana, F.; Jarrín-v, P.; Donoso, N.; Romero-Benavides, J.C. “Horchata” Drink in Southern Ecuador: Medicinal Plants and People’s Wellbeing. J. Ethnobiol. Ethnomed. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Guevara, M.; Tejera, E.; Iturralde, G.A.; Jaramillo-Vivanco, T.; Granda-Albuja, M.G.; Granja-Albuja, S.; Santos-Buelga, C.; González-Paramás, A.M.; Álvarez-Suarez, J.M. Anti-Inflammatory Effect of the Medicinal Herbal Mixture Infusion, Horchata, from Southern Ecuador against LPS-Induced Cytotoxic Damage in RAW 264.7 Macrophages. Food Chem. Toxicol. 2019, 131, 110594. [Google Scholar] [CrossRef]
- Guevara, M.; Proaño, A.; Tejera, E.; Ballesteros, I.; Sánchez, M.E.; Granda-Albuja, M.G.; Freire, B.; Chisaguano, A.M.; Debut, A.; Vizuete, K.; et al. Protective Effect of the Medicinal Herb Infusion “Horchata” against Oxidative Damage in Cigarette Smokers: An Ex Vivo Study. Food Chem. Toxicol. 2020, 143, 111538. [Google Scholar] [CrossRef] [PubMed]
- Tejera, E.; Pérez-Castillo, Y.; Toscano, G.; Noboa, A.L.; Ochoa-Herrera, V.; Giampieri, F.; Álvarez-Suarez, J.M. Computational Modeling Predicts Potential Effects of the Herbal Infusion “Horchata” against COVID-19. Food Chem. 2022, 366, 130589. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural Products: A Lead for Drug Discovery and Development. Phyther. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Srichok, J.; Yingbun, N.; Kowawisetsut, T.; Kornmatitsuk, S.; Suttisansanee, U.; Temviriyanukul, P.; Chantong, B. Synergistic Antibacterial and Anti-Inflammatory Activities of Ocimum Tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens. Antibiotics 2022, 11, 510. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Vidari, G. Poorly Investigated Ecuadorian Medicinal Plants. Plants 2022, 11, 1590. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://apps.who.int/iris/handle/10665/311820 (accessed on 20 August 2022).
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical Lab Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Levison, M.E. Pharmacodynamics of Antimicrobial Drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef]
- Chang, J.; Lane, M.; Yang, M.; Heinrich, M. A Hexa-Herbal TCM Decoction Used to Treat Skin Inflammation: An LC-MS-Based Phytochemical Analysis. Planta Med. 2016, 82, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Tudella, J.; Nunes, F.M.; Paradela, R.; Evtuguin, D.V.; Domingues, P.; Amado, F.; Coimbra, M.A.; Barros, A.I.R.N.A.; Domingues, M.R.M. Oxidation of Mannosyl Oligosaccharides by Hydroxyl Radicals as Assessed by Electrospray Mass Spectrometry. Carbohydr. Res. 2011, 346, 2603–2611. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and Characterization of Polyphenol Type-A Polymers from Cinnamon with Insulin-like Biological Activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Mouls, L.; Mazauric, J.-P.; Sommerer, N.; Fulcrand, H.; Mazerolles, G. Comprehensive Study of Condensed Tannins by ESI Mass Spectrometry: Average Degree of Polymerisation and Polymer Distribution Determination from Mass Spectra. Anal. Bioanal. Chem. 2011, 400, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, R.; Guan, Z.; Chen, A.; Li, W. Ultra-Performance LC Separation and Quadrupole Time-of-Flight MS Identification of Major Alkaloids in Plumula Nelumbinis. Phytochem. Anal. 2014, 25, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-Y.; Yu, J.-W.; Lu, F.-L.; Lin, M.-C.; Cheng, H.-F. Differentiating Parts of Cinnamomum Cassia Using LC-QTOF-MS in Conjunction with Principal Component Analysis. Biomed. Chromatogr. 2016, 30, 1449–1457. [Google Scholar] [CrossRef]
- Huynh, H.T.; Tsai, S.-T.; Hsu, P.-J.; Biswas, A.; Phan, H.T.; Kuo, J.-L.; Ni, C.-K.; Chiu, C. Collision-Induced Dissociation of Na + -Tagged Ketohexoses: Experimental and Computational Studies on Fructose. Phys. Chem. Chem. Phys. 2022, 24, 20856–20866. [Google Scholar] [CrossRef]
- Taylor, V.F.; March, R.E.; Longerich, H.P.; Stadey, C.J. A Mass Spectrometric Study of Glucose, Sucrose, and Fructose Using an Inductively Coupled Plasma and Electrospray Ionization. Int. J. Mass Spectrom. 2005, 243, 71–84. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Pastene-Navarrete, E. Green Extraction of Alkaloids and Polyphenols from Peumus Boldus Leaves with Natural Deep Eutectic Solvents and Profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- de Lima, B.; da Silva, F.; Soares, E.; de Almeida, R.; da Silva-Filho, F.; Barison, A.; Costa, E.; Koolen, H.; de Souza, A.; Pinheiro, M.L. Integrative Approach Based on Leaf Spray Mass Spectrometry, HPLC-DAD-MS/MS, and NMR for Comprehensive Characterization of Isoquinoline-Derived Alkaloids in Leaves of Onychopetalum Amazonicum R. E. Fr. J. Braz. Chem. Soc. 2020, 31, 79–89. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Srivastava, M.; Arya, K.R.; Kumar, B. Rapid Screening and Distribution of Bioactive Compounds in Different Parts of Berberis Petiolaris Using Direct Analysis in Real Time Mass Spectrometry. J. Pharm. Anal. 2015, 5, 332–335. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Xue, X.; Zhang, Y.; Xiao, H.; Liang, X. Rapid Identification of Polyphenol C-Glycosides from Swertia Franchetiana by HPLC--ESI-MS--MS. J. Chromatogr. Sci. 2009, 47, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, H.; Nilsson, P.; Jansson, H.-B.; Odham, G. Characterization and Determination of Prehelminthosporol, a Toxin from the Plant Pathogenic Fungus Bipolaris Sorokiniana, Using Liquid Chromatography/Mass Spectrometry. J. Microbiol. Methods 1991, 13, 259–269. [Google Scholar] [CrossRef]
- Shui, G.; Wong, S.P.; Leong, L.P. Characterization of Antioxidants and Change of Antioxidant Levels during Storage of Manilkara zapota L. J. Agric. Food Chem. 2004, 52, 7834–7841. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, F.; Ramachandran, V.N.; Smyth, T.J.P.; Smyth, W.F.; Brooks, P. An Investigation of Bioactive Phytochemicals in the Leaves of Melicope Vitiflora by Electrospray Ionisation Ion Trap Mass Spectrometry. Anal. Chim. Acta 2009, 634, 115–120. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Martiskainen, O.; Seif el-Din, S.H.; Sabra, A.-N.A.; Hammam, O.A.; El-Lakkany, N.M. Protective Effect of Pelargonium graveolens against Carbon Tetrachloride-Induced Hepatotoxicity in Mice and Characterization of Its Bioactive Constituents by HPLC–PDA–ESI–MS/MS Analysis. Med. Chem. Res. 2015, 24, 1438–1448. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.-P. Biological Activity of Ellagitannins: Effects as Anti-Oxidants, pro-Oxidants and Metal Chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef]
- Silva, L.N.; Rigo, G.V.; Silva, D.B.; Carollo, C.A.; Trentin, D.S.; Silva, M.V.; Tasca, T.; Macedo, A.J. Hydrolyzable Tannins from Poincianella (Caesalpinia) Microphylla Fruits: Metabolite Profiling and Anti-Trichomonas Vaginalis Activity. Food Res. Int. 2020, 134, 109236. [Google Scholar] [CrossRef]
- Wyrepkowski, C.; Gomes da Costa, D.; Sinhorin, A.; Vilegas, W.; De Grandis, R.; Resende, F.; Varanda, E.; dos Santos, L. Characterization and Quantification of the Compounds of the Ethanolic Extract from Caesalpinia Ferrea Stem Bark and Evaluation of Their Mutagenic Activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [Green Version]
- Llorent-Martínez, E.J.; Gouveia, S.; Castilho, P.C. Analysis of Phenolic Compounds in Leaves from Endemic Trees from Madeira Island. A Contribution to the Chemotaxonomy of Laurisilva Forest Species. Ind. Crops Prod. 2015, 64, 135–151. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Lc-Ms/Ms-Qtof Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Nowak, M.; de Graft-Johnson, J.; Padula, G.; Bialasiewicz, P.; Markowski, J.; et al. Consumption of Strawberries on a Daily Basis Increases the Non-Urate 2,2-Diphenyl-1-Picryl-Hydrazyl (DPPH) Radical Scavenging Activity of Fasting Plasma in Healthy Subjects. J. Clin. Biochem. Nutr. 2014, 55, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits Consumed in the Western Coastal Region of Ecuador as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastidas, C.A.; Villacrés-Granda, I.; Navarrete, D.; Monsalve, M.; Coral-Almeida, M.; Cifuentes, S.G. Antibiotic Susceptibility Profile and Prevalence of MecA and LukS-PV/LukF-PV Genes in Staphylococcus aureus Isolated from Nasal and Pharyngeal Sources of Medical Students in Ecuador. Infect. Drug Resist. 2019, 12, 2553–2560. [Google Scholar] [CrossRef] [Green Version]
- Villacrés-Granda, I.; Coello, D.; Proaño, A.; Ballesteros, I.; Roubik, D.W.; Jijón, G.; Granda-Albuja, G.; Granda-Albuja, S.; Abreu-Naranjo, R.; Maza, F.; et al. Honey Quality Parameters, Chemical Composition and Antimicrobial Activity in Twelve Ecuadorian Stingless Bees (Apidae: Apinae: Meliponini) Tested against Multiresistant Human Pathogens. Lwt 2021, 140, 110737. [Google Scholar] [CrossRef]
- García-Tenesaca, M.; Navarrete, E.S.; Iturralde, G.A.; Villacrés Granda, I.M.; Tejera, E.; Beltrán-Ayala, P.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Influence of Botanical Origin and Chemical Composition on the Protective Effect against Oxidative Damage and the Capacity to Reduce in Vitro Bacterial Biofilms of Monofloral Honeys from the Andean Region of Ecuador. Int. J. Mol. Sci. 2018, 19, 45. [Google Scholar] [CrossRef] [Green Version]
- Moser, K.A.; Zhang, L.; Spicknall, I.; Braykov, N.P.; Levy, K.; Marrs, C.F.; Foxman, B.; Trueba, G.; Cevallos, W.; Goldstick, J.; et al. The Role of Mobile Genetic Elements in the Spread of Antimicrobial-Resistant Escherichia coli from Chickens to Humans in Small-Scale Production Poultry Operations in Rural Ecuador. Am. J. Epidemiol. 2018, 187, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Parente, E.; Hill, C. A Comparison of Factors Affecting the Production of Two Bacteriocins from Lactic Acid Bacteria. J. Appl. Bacteriol. 1992, 73, 290–298. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical Lab Standards Institute: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Sornsenee, P.; Chatatikun, M.; Mitsuwan, W.; Kongpol, K.; Kooltheat, N.; Sohbenalee, S.; Pruksaphanrat, S.; Mudpan, A.; Romyasamit, C. Lyophilized Cell-Free Supernatants of Lactobacillus Isolates Exhibited Antibiofilm, Antioxidant, and Reduces Nitric Oxide Activity in Lipopolysaccharide- Stimulated RAW 264.7 Cells. PeerJ 2021, 9, e12586. [Google Scholar] [CrossRef]
- Patel, B.; Siddiqui, A.; Hamadou, W.; Awadelkareem, A.; Ashraf, S.; Alreshidi, M.; Snoussi, M.; Rizvi, S.; Bardakci, F. Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Mahmud, R. Chemical Analysis, Inhibition of Biofilm Formation and Biofilm Eradication Potential of Euphorbia hirta L. against Clinical Isolates and Standard Strains. BMC Complement. Altern. Med. 2013, 13, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmawatie, M.D.; Wibawa, T.; Lisdiyanti, P.; Pratiwi, W.R.; Mustofa; Das, P.; Mukherjee, S.; Sen, R.; Yang, X.P.; Ma, L.Z.; et al. Evaluation of Crystal Violet Decolorization Assay and Resazurin Microplate Assay for Antimycobacterial Screening. J. Appl. Microbiol. 2019, 5, e02263. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://cran.r-project.org/package=rstatix (accessed on 10 July 2022).
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2005, 10, 813–829. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef] [PubMed]
- Buru, A.S.; Pichika, M.R.; Neela, V.; Mohandas, K. In Vitro Antibacterial Effects of Cinnamomum Extracts on Common Bacteria Found in Wound Infections with Emphasis on Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 2014, 153, 587–595. [Google Scholar] [CrossRef]
- Chang, S.; Chen, P.; Chang, S. Antibacterial Activity of Leaf Essential Oils and Their Constituents from Cinnamomum Osmophloeum. J. Ethnopha 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Shangguan, W.; Chen, S.; Zhong, Q. Antibiofilm Activities of the Cinnamon Extract against Vibrio parahaemolyticus and Escherichia coli. Arch. Microbiol. 2021, 203, 125–135. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Mnif, W.; Dhifi, W.; Jelali, N.; Baaziz, H.; Hadded, A.; Hamdi, N. Characterization of Leaves Essential Oil of Pelargonium graveolens Originating from Tunisia: Chemical Composition, Antioxidant and Biological Activities. J. Essent. Oil-Bearing Plants 2011, 14, 761–769. [Google Scholar] [CrossRef]
- Ben Slima, A.; Ben Ali, M.; Barkallah, M.; Traore, A.I.; Boudawara, T.; Allouche, N.; Gdoura, R. Antioxidant Properties of Pelargonium graveolens L’Her Essential Oil on the Reproductive Damage Induced by Deltamethrin in Mice as Compared to Alpha-Tocopherol. Lipids Health Dis. 2013, 12, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmen, G.; Hancu, G. Antimicrobial and Antifungal Activity of Pelargonium roseum Essential Oils. Adv. Pharm. Bull. 2014, 4, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Moutaouafiq, S.; Farah, A.; Ez zoubi, Y.; Ghanmi, M.; satrani, B.; Bousta, D. Antifungal Activity of Pelargonium graveolens Essential Oil and Its Fractions Against Wood Decay Fungi. J. Essent. Oil-Bearing Plants 2019, 22, 1104–1114. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-Inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Roth, G. Composition of the Essential Oils of Pelargonium odoratissimum, P. exstipulatum, and P. xfragrans (Geraniaceae) and Their Bioactivity. Flavour Fragr. J. 2000, 15, 391–394. [Google Scholar] [CrossRef]
- Andrade, M.A.; Cardoso, M.G.; Batista, L.R.; Freire, J.M.; Nelson, D.L. Antimicrobial Activity and Chemical Composition of Essential Oil of Pelargonium odoratissimum. Rev. Bras. Farmacogn. 2011, 21, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial Activity of Phenolic Acids against Commensal, Probiotic and Pathogenic Bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schneider, S.; Stintzing, F.C.; Carle, R.; Reichling, J. Efficacy of an Aqueous Pelargonium sidoides Extract against Herpesvirus. Phytomedicine 2008, 15, 1108–1116. [Google Scholar] [CrossRef]
- Panara, A.; Aalizadeh, R.; Thomaidis, N.S. Chemical Characterisation of Pelargonium sidoides Root Based on LC-QToF-MS Non-target Screening Strategies. Phytochem. Anal. 2022, 33, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulou, C.; Christopoulou, S.D.; Hahalis, P.; Kotsalou, C.; Lamari, F.N.; Vantarakis, A. Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity. Pathogens 2021, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial Properties and Major Bioactive Components of Cinnamon Stick (Cinnamomum burmannii): Activity against Foodborne Pathogenic Bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Mateos-Martín, M.L.; Fuguet, E.; Quero, C.; Pérez-Jiménez, J.; Torres, J.L. New Identification of Proanthocyanidins in Cinnamon (Cinnamomum zeylanicum L.) Using MALDI-TOF/TOF Mass Spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Cai, X.Y.; He, Y.Y.; Lu, M.Y.; Liu, S.; Wang, W.X.; Li, Z.H.; Ai, H.L.; Feng, T. Seco-Sativene and Seco-Longifolene Sesquiterpenoids from Cultures of Endophytic Fungus Bipolaris Eleusines. Nat. Prod. Bioprospect. 2017, 7, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M.A.; Gōng, J.; Mehmood, S.; Yuán, Y.; Gǒng, W. Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa? Front. Plant Sci. 2021, 12, 3060. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Maurya, A.L.; Verma, V.C.; Kumar, A.; Gond, S.K.; Mishra, A. Diversity and Antimicrobial Activity of Endophytic Fungal Community Isolated from Medicinal Plant Cinnamomum camphora. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2012, 82, 557–565. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.; Mattila-sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Wright, A. Von Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 8561, 3590–3595. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as a Promising Component of Biomaterials-A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Newman, M.; Gibby, M. The Application of Leaf Phenolic Evidence for Systematic Studies within the Genus Pelargonium (Geraniaceae). Biochem. Syst. Ecol. 2000, 28, 119–132. [Google Scholar] [CrossRef]
- Neumann, N.; Honke, M.; Povydysh, M.; Guenther, S.; Schulze, C. Evaluating Tannins and Flavonoids from Traditionally Used Medicinal Plants with Biofilm Inhibitory Effects against MRGN E. coli. Molecules 2022, 27, 2284. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Hamilton-Miller, J.M.T.; Hara, Y.; Nagaoka, Y.; Kumagai, A.; Uesato, S.; Taylor, P.W. Anti-Staphylococcus aureus Activity and Oxacillin Resistance Modulating Capacity of 3-O-Acyl-Catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The β-Lactam-Resistance Modifier (-)-Epicatechin Gallate Alters the Architecture of the Cell Wall of Staphylococcus Aureas. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus Following Treatment with the Antibacterial Flavonol Galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the Antibacterial Activity of 3-O-Octanoyl-(-)-Epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus Officinalis, Ocimum Basilicum and Teucrium Chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Masoumian, M.; Zandi, M. Antimicrobial Activity of Some Medicinal Plants against Multidrug Resistant Skin Pathogens. J. Med. Plants Res. 2011, 5, 3856–3860. [Google Scholar] [CrossRef]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; De Carvalho, M.G.; Catunda, F.E.A. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giongo, J.L.; de Almeida Vaucher, R.; Fausto, V.P.; Quatrin, P.M.; Lopes, L.Q.S.; Santos, R.C.V.; Gündel, A.; Gomes, P.; Steppe, M. Anti-Candida Activity Assessment of Pelargonium graveolens Oil Free and Nanoemulsion in Biofilm Formation in Hospital Medical Supplies. Microb. Pathog. 2016, 100, 170–178. [Google Scholar] [CrossRef]
- Didehdar, M.; Chegini, Z.; Tabaeian, S.P.; Razavi, S.; Shariati, A. Cinnamomum: The New Therapeutic Agents for Inhibition of Bacterial and Fungal Biofilm-Associated Infection. Front. Cell. Infect. Microbiol. 2022, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Kot, B.; Wierzchowska, K.; Grużewska, A.; Lohinau, D. The Effects of Selected Phytochemicals on Biofilm Formed by Five Methicillin-Resistant Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 1299–1302. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Nisha, P.; Murugan, R.; Thamotharan, S.; Percino, M.J.; Subramani, P.; Nithyanand, P. Essential Oils from Unexplored Aromatic Plants Quench Biofilm Formation and Virulence of Methicillin Resistant Staphylococcus aureus. Microb. Pathog. 2018, 122, 162–173. [Google Scholar] [CrossRef]
- Lakshmana Prabu, S.; Umamaheswari, A.; Grace Felciya, S.J. Investigation on the Biofilm Eradication Potential of Selected Medicinal Plants against Methicillin-Resistant Staphylococcus aureus. Biotechnol. Rep. 2020, 28, e00523. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Lagha, R.; Gaber, A. Biofilm Inhibition and Eradication Properties of Medicinal Plant Essential Oils against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory Activity of Yarrow Essential Oil on Listeria Planktonic Cells and Biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Bjarnsholt, T. Introduction to Biofilms. In Biofilm Infections; Bjarnsholt, T., Moser, C., Jensen, P.Ø., Høiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 1–9. ISBN 9781441960832. [Google Scholar]
- Miyaue, S.; Suzuki, E.; Komiyama, Y.; Kondo, Y.; Morikawa, M.; Maeda, S. Bacterial Memory of Persisters: Bacterial Persister Cells Can Retain Their Phenotype for Days or Weeks after Withdrawal from Colony-Biofilm Culture. Front. Microbiol. 2018, 9, 1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, G.A.; Rossatto, F.C.P.; Medeiros, A.W.; Correa, A.P.F.; Brandelli, A.; Frazzon, A.P.G.; Motta, A.D.S. Evaluation Antibacterial and Antibiofilm Activity of the Antimicrobial Peptide P34 against Staphylococcus aureus and Enterococcus faecalis. An. Acad. Bras. Cienc. 2018, 90, 73–84. [Google Scholar] [CrossRef] [PubMed]


| N | Scientific Name | TPC (mg GAE per g DE) | TFC (mg CE per g DE) | TAC (μmol TEq per g DE) | |
|---|---|---|---|---|---|
| FRAP | DPPH | ||||
| 1 | Amaranthus hybridus | 28.7 ± 0.9 | 10.9 ± 1.0 | 87.1 ± 5.5 | 136.3 ± 5.0 |
| 2 | Ocimum tenuiflorum | 36.2 ± 1.8 | 14.9 ± 0.3 | 155.6 ± 6.5 | 177.8 ± 10.5 |
| 3 | Ocimum basilicum L. | 91.3 ± 2.9 | 87.4 ± 2.0 | 705.0 ± 32.2 | 697.8 ± 11.9 |
| 4 | Borago officinalis L. | 48.8 ± 2.6 | 31.9 ± 1.5 | 238.7 ± 9.0 | 263.6 ± 25.9 |
| 5 | Aloysia triphylla (L’Hér.) Britton | 98.3 ± 1.9 | 77.6 ± 3.4 | 622.9 ± 7.3 | 761.3 ± 33.1 |
| 6 | Adiantum concinnum Humb. and Bonpl. ex Wild. | 105.2 ± 3.0 | 78.8 ± 2.1 | 438.8 ± 18.9 | 1036.3 ± 91.2 |
| 7 | Equisetum bogotense Kunth | 224.4 ± 6.3 | 19.9 ± 0.6 | 171.6 ± 4.1 | 228.9 ± 19.5 |
| 8 | Cinnamomum sp. | 382.1 ± 28.1 | 180.5 ± 19.9 | 1471.3 ± 18.1 | 2894.3 ± 272.6 |
| 9 | Aerva sanguinolenta L. (Blume). | 50.3 ± 1.8 | 23.0 ± 1.3 | 192.9 ± 2.2 | 284.0 ± 21.7 |
| 10 | Stevia rebaudiana (Bertoni) Bertoni | 149.5 ± 9.7 | 98.6 ± 2.9 | 601.3 ± 26.6 | 621.8 ± 79.6 |
| 11 | Cymbopogon citratus (DC.) Stapf | 31.6 ± 3.4 | 14.1 ± 0.5 | 117.2 ± 4.7 | 164.3 ± 10.5 |
| 12 | Citrus x aurantium L. | 71.3 ± 0.9 | 34.7 ± 0.7 | 256.6 ± 2.2 | 341.0 ± 25.1 |
| 13 | Plantago major L. | 35.0 ± 1.5 | 17.6 ± 0.2 | 123.2 ± 6.1 | 183.5 ± 7.6 |
| 14 | Pelargonium odoratissimum (L.) L’Hér. | 258.2 ± 14.4 | 31.0 ± 1.4 | 901.8 ± 38.7 | 2777.9 ± 17.1 |
| 15 | Althaea officinalis L. | 33.8 ± 1.1 | 5.9 ± 0.2 | 71.0 ± 4.9 | 79.9 ± 6.6 |
| 16 | Matricaria chamomilla L. | 32.5 ± 1.7 | 9.9 ± 0.2 | 107.4 ± 4.5 | 154.9 ± 1.1 |
| 17 | Mentha x piperita L. | 52.2 ± 0.9 | 45.4 ± 1.0 | 198.5 ± 6.7 | 245.7 ± 23.7 |
| 18 | Origanum vulgare L. | 96.1 ± 4.1 | 56.1 ± 2.8 | 507.0 ± 39.7 | 768.5 ± 12.9 |
| 19 | Fuchsia loxensis Kunth | 67.5 ± 1.0 | 29.8 ± 1.1 | 317.6 ± 8.0 | 533.4 ± 44.9 |
| 20 | Melissa officinalis L. | 299.6 ± 10.3 | 256.4 ± 3.3 | 1582.4 ± 67.0 | 1965.2 ± 84.3 |
| 21 | Viola odorata L. | 74.0 ± 1.2 | 31.9 ± 1.0 | 462.9 ± 34.8 | 568.1 ± 47.7 |
| N | Plant Scientific Name | Susceptible Bacterial Strains (Inhibition Zone Diameter around Circular Well in mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E. faecium | E. faecalis | S. aureus | K. pneumoniae | A. baumannii | P. aeruginosa | E. cloacae | E. coli | ||
| 1 | Amaranthus hybridus | - | - | - | - | - | - | - | - |
| 2 | Ocimum tenuiflorum | - | - | - | - | - | - | - | - |
| 3 | Ocimum basilicum L. | - | 10 | 6 | - | - | - | - | - |
| 4 | Borago officinalis L. | - | 6 | 2 | - | - | - | - | - |
| 5 | Aloysia triphylla (L’Hér.) Britton | - | - | 2 | - | 4 | - | - | - |
| 6 | Adiantum concinnum Humb. and Bonpl. ex Wild | - | 6 | 10 | - | 7 | - | - | - |
| 7 | Equisetum bogotense Kunth | - | - | - | - | - | - | - | - |
| 8 | Cinnamomum sp. | - | - | 17 | - | 5 | - | 5 | - |
| 9 | Aerva sanguinolenta L. (Blume) | - | - | - | - | - | - | - | - |
| 10 | Stevia rebaudiana (Bertoni) Bertoni | - | - | 7 | - | - | - | - | - |
| 11 | Cymbopogon citratus (DC.) Stapf | - | - | - | - | - | - | - | 6 |
| 12 | Citrus x aurantium L. | - | - | - | - | - | - | - | - |
| 13 | Plantago major L. | - | - | 8 | - | - | - | - | - |
| 14 | Pelargonium odoratissimum (L.) L’Hér. | - | - | 15 | 3 | - | - | - | 10 |
| 15 | Althaea officinalis L. | - | - | - | - | - | - | - | - |
| 16 | Matricaria chamomilla L. | - | - | 7 | - | - | - | - | - |
| 17 | Mentha x piperita L. | - | 6 | 9 | - | - | - | - | - |
| 18 | Origanum vulgare L. | - | 9 | 11 | - | - | - | - | - |
| 19 | Fuchsia loxensis Kunth | - | - | - | - | - | - | - | - |
| 20 | Melissa officinalis L. | - | - | - | - | - | - | - | - |
| 21 | Viola odorata L. | - | - | - | - | - | - | - | - |
| Negative control | - | - | - | - | - | - | - | - | |
| N | PlantScientific Name | Resistant Bacterial Strains (Inhibition Zone Diameter around Circular Well in mm) | |||||
|---|---|---|---|---|---|---|---|
| MRSA 333 | E. faecalis INSPI 032 | K. pneumoniae KPC 609803 | E. coli ESBL | E. coli INSPI 033 | A. baumannii ATCC1605 | ||
| 1 | Ocimum basilicum L. | - | - | NT | NT | NT | NT |
| 2 | Borago officinalis L. | - | - | NT | NT | NT | NT |
| 3 | Aloysia triphylla (L’Hér.) Britton | - | NT | NT | NT | NT | - |
| 4 | Adiantum concinnum Humb. and Bonpl. ex Wild | - | - | NT | NT | NT | - |
| 5 | Cinnamomum sp. | 14 | NT | NT | NT | NT | - |
| 6 | Stevia rebaudiana (Bertoni) Bertoni | 7 | NT | NT | NT | NT | - |
| 7 | Cymbopogon citratus (DC.) Stapf | NT | NT | NT | - | - | NT |
| 8 | Plantago major L. | - | NT | NT | NT | NT | NT |
| 9 | Pelargonium odoratissimum (L.) L’Hér. | 4 | NT | - | - | - | NT |
| 10 | Matricaria chamomilla L. | - | NT | NT | NT | NT | NT |
| 11 | Mentha x piperita L. | - | - | NT | NT | NT | NT |
| 12 | Origanum vulgare L. | - | - | NT | NT | NT | NT |
| Negative control | - | - | - | - | - | - | |
| N | Plant Scientific Name | Bacterial Strains MIC; MBC (Values Are in µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E. faecalis | S. aureus | K. pneumoniae | A. baumannii | E. cloacae | E. coli | MRSA 333 | E. faecalis INSPI 032 | ||
| 1 | Ocimum basilicum L. | >1000 | >1000 | NT | NT | NT | NT | NT | NT |
| 2 | Borago officinalis L. | >1000 | >1000 | NT | NT | NT | NT | NT | NT |
| 3 | Aloysia triphylla (L’Hér.) Britton | NT | >1000 | NT | >1000 | NT | NT | NT | NT |
| 4 | Adiantum concinnum Humb. and Bonpl. ex Wild | >1000 | >1000 | NT | >1000 | NT | NT | NT | NT |
| 5 | Cinnamomum sp. | NT | 250; 250 | NT | >1000 | >1000 | NT | 250; 500 | NT |
| 6 | Stevia rebaudiana (Bertoni) Bertoni | NT | >1000 | NT | NT | NT | NT | >1000 | NT |
| 7 | Cymbopogon citratus (DC.) Stapf | NT | NT | NT | NT | NT | >1000 | NT | NT |
| 8 | Plantago major L. | NT | >1000 | NT | NT | NT | NT | NT | NT |
| 9 | Pelargonium odoratissimum (L.) L’Hér. | NT | 500; 1000 | >1000 | NT | NT | >1000 | >1000 | NT |
| 10 | Matricaria chamomilla L. | NT | >1000 | NT | NT | NT | NT | NT | NT |
| 11 | Mentha x piperita L. | >1000 | >1000 | NT | NT | NT | NT | NT | NT |
| 12 | Origanum vulgare L. | >1000 | >1000 | NT | NT | NT | NT | NT | NT |
| - | Ciprofloxacin | 1.5 | 0.38 | 0.15 | 0.75 | <0.09 | <0.09 | NT | NT |
| N | Common Name | Scientific Name | Part of the Plant |
|---|---|---|---|
| 1 | Ataco | Amaranthus hybridus | Flower |
| 2 | Albahaca negra o dulce | Ocimum tenuiflorum | Leaf, stalk |
| 3 | Albahaca blanca o salada | Ocimum basilicum L. | Leaf, stalk |
| 4 | Borraja | Borago officinalis L. | Plant without root |
| 5 | Cedrón | Aloysia triphylla (L’Hér.) Britton | Leaf |
| 6 | Culantrillo | Adiantum concinnum Humb. and Bonpl. ex Wild. | Leaf |
| 7 | Cola de caballo | Equisetum bogotense Kunth | Branch |
| 8 | Canela | Cinnamomum sp. | Bark |
| 9 | Escancel | Aerva sanguinolenta L. (Blume). | Plant without root |
| 10 | Stevia | Stevia rebaudiana (Bertoni) Bertoni | Leaf |
| 11 | Hierba luisa | Cymbopogon citratus (DC.) Stapf | Leaf |
| 12 | Hoja de naranja | Citrus x aurantium L. | Leaf |
| 13 | Llantén | Plantago major L. | Plant without root |
| 14 | Malva olorosa | Pelargonium odoratissimum (L.) L’Hér. | Leaf |
| 15 | Malva blanca | Althaea officinalis L. | Leaf |
| 16 | Manzanilla | Matricaria chamomilla L. | Plant without root |
| 17 | Menta | Mentha x piperita L. | Leaf, stalk |
| 18 | Orégano dulce | Origanum vulgare L. | Leaf |
| 19 | Pena pena | Fuchsia loxensis Kunth | Plant without root |
| 20 | Toronjil | Melissa officinalis L. | Leaf, stalk |
| 21 | Violeta | Viola odorata L. | Flower, leaf |
| N | Microorganisms | Source | Available Information | Reference |
|---|---|---|---|---|
| 1 | Enterococcus faecium ATCC27270 | Enterococcus faecium (Orla-Jensen) Schleifer and Kilpper-Balz (ATCC27270) | Isolate contains enterococcal bacteriocins, and it is a whole-genome sequenced clinical isolate. | ATCC |
| 2 | Enterococcus faecalis ATCC29212 | Enterococcus faecalis (Andrewes and Horder) Schleifer and Kilpper-Balz (ATCC29212) | The isolate was obtained from a human urine sample, and it is a whole-genome sequenced clinical isolate. | ATCC |
| 3 | Staphylococcus aureus ATCC25923 | Staphylococcus aureus subsp. aureus Rosenbach (ATCC25923) | The strain was obtained in Seattle in 1945, and it is a whole-genome sequenced clinical isolate. | ATCC |
| 4 | Klebsiella pneumoniae ATCC700603 | Klebsiella quasipneumoniae Brisse et al. (ATCC700603) | It is a whole-genome sequenced bacterium that was isolated from the urine of a hospitalised patient in Richmond, Virginia. This bacterium produces beta-lactamase SHV-18 and can be used as a CLSI quality control strain for antimicrobial susceptibility testing. | ATCC |
| 5 | Acinetobacter baumannii ATCC19606 | Acinetobacter baumannii Bouvet and Grimont (ATCC19606) | The isolate was obtained from a human urine sample, and it is a whole-genome sequenced clinical isolate that can be used as a quality control strain for antimicrobial susceptibility testing. | ATCC |
| 6 | Acinetobacter baumannii ATCC1605 | Acinetobacter baumannii (ATCCBAA-1605) | The isolate was obtained from a human sputum of military personnel returning from Afghanistan entering a Canadian hospital and can be used for antimicrobial resistance research or drug development. | ATCC |
| 7 | Pseudomonas aeruginosa ATCC27853 | Pseudomonas aeruginosa (Schroeter) Migula (ATCC27853) | The isolate was obtained from a hospital blood specimen in 1971, and this whole-genome sequenced bacterial strain has applications in susceptibility testing. | ATCC |
| 8 | Enterobacter cloacae ATCC23355 | Enterobacter cloacae subsp. cloacae (Jordan) Hormaeche and Edwards, subsp. nov. (ATCC23355) | Isolate produces cephalosporinase beta-lactamase II, and this whole-genome sequenced bacterial strain is a well-known bacteriophage host. | ATCC |
| 9 | Escherichia coli ATCC25922 | Escherichia coli (Migula) Castellani and Chalmers (ATCC25922) | Escherichia coli human isolate strain Seattle 1946 is a whole-genome sequenced quality control strain that does not produce verotoxin and can be used as a CLSI control strain for antimicrobial susceptibility testing. | ATCC |
| 10 | Methicillin-resistant Staphylococcus aureus 333 | Universidad de Las Américas (UDLA) | Isolated strains obtained from nasal and pharyngeal sources in Ecuadorian patients. | [49,50] |
| 11 | Klebsiella pneumoniae carbapenemase (KPC)-producing 609803 | UDLA | The isolate was obtained from human samples at the Research Laboratories of the Universidad de Las Américas (UDLA). | [51] |
| 12 | Vancomycin-Resistant Enterococcus faecalis INSPI 032 | Instituto Nacional de Investigación en Salud Pública (INSPI) | The isolate was obtained from human samples, and it was donated by INSPI. | INSPI |
| 13 | Metallo-β-lactamase (MBL) Escherichia coli INSPI 033 | INSPI | The isolate was obtained from human sample, and it was donated by the National Institute for Research in Public Health (INSPI) in Ecuador. | INSPI |
| 14 | Escherichia coli extended-spectrum beta-lactamases (ESBLs) | Institute of Microbiology at Universidad San Francisco de Quito (IM-USFQ) | The isolate was obtained from human faecal sample at the Clinical Microbiology Laboratory of the IM-USFQ. | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Soto, P.; Celi, D.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Cinnamomum sp. and Pelargonium odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants. Molecules 2023, 28, 693. https://doi.org/10.3390/molecules28020693
Fernandez-Soto P, Celi D, Tejera E, Alvarez-Suarez JM, Machado A. Cinnamomum sp. and Pelargonium odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants. Molecules. 2023; 28(2):693. https://doi.org/10.3390/molecules28020693
Chicago/Turabian StyleFernandez-Soto, Paulina, Diana Celi, Eduardo Tejera, José Miguel Alvarez-Suarez, and António Machado. 2023. "Cinnamomum sp. and Pelargonium odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants" Molecules 28, no. 2: 693. https://doi.org/10.3390/molecules28020693
APA StyleFernandez-Soto, P., Celi, D., Tejera, E., Alvarez-Suarez, J. M., & Machado, A. (2023). Cinnamomum sp. and Pelargonium odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants. Molecules, 28(2), 693. https://doi.org/10.3390/molecules28020693