Triterpenoid and Steroid Content of Lipophilic Extracts of Selected Medicinal Plants of the Mediterranean Region
Abstract
:1. Introduction
2. Results
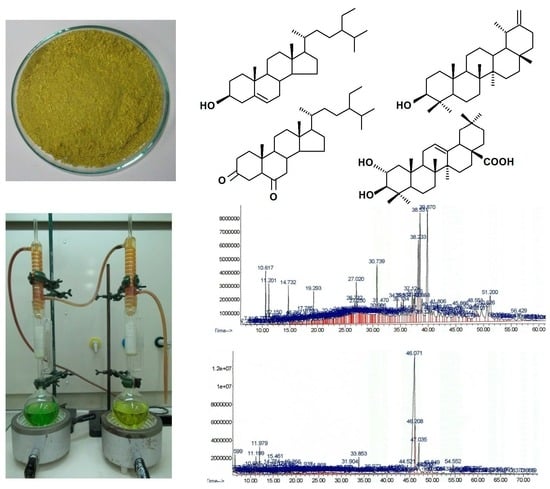
2.1. Identification of Steroids and Triterpenoids in Obtained Extracts
2.2. The Content of Steroids and Triterpenoids in Cistus ladanifer and Cistus monspeliensis
2.3. The Content of Steroids and Triterpenoids in Erica arborea
2.4. The Content of Steroids and Triterpenoids in Globularia alypum Leaves
2.5. The Content of Steroids and Triterpenoids in Pistacia lentiscus Leaves
2.6. The Content of Steroids and Triterpenoids in Rhamnus alaternus Leaves
2.7. Determination of Radical Scavenging Activity of Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Fractionation of Diethyl Ether Extracts
4.4. Derivatization of Triterpenoid Acids
4.5. Identification and Quantification of Steroids by Gas Chromatography-Mass Spectrometry
4.6. Determination of Free Radical Scavenging Activity
4.7. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aidi Wannes, W.; Saidani Tounsi, M.; Marzouk, B. A review of Tunisian medicinal plants with anticancer activity. J. Complement. Integr. Med. 2018, 15, 20170052. [Google Scholar] [CrossRef] [PubMed]
- Adigew, M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. Current uses and knowledge of medicinal plants in the Autonomous Community of Madrid (Spain): A descriptive cross-sectional study. BMC Complement. Med. Ther. 2020, 20, 306. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Wrońska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The synergistic effect of triterpenoids and flavonoids—New approaches for treating bacterial infections? Molecules 2022, 27, 847. [Google Scholar] [CrossRef] [PubMed]
- Tonga, J.L.; Kamdem, M.H.K.; Pagna, J.I.M.; Fonkui, T.Y.; Tata, C.M.; Fotsing, M.C.D.; Nkengfack, E.A.; Mmutlane, E.M.; Ndinteh, D.T. Antibacterial activity of flavonoids and triterpenoids isolated from the stem bark and sap of Staudtia kamerunensis Warb. (Myristicaceae). Arab. J. Chem. 2022, 15, 104150. [Google Scholar] [CrossRef]
- Fang, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef] [PubMed]
- Suryamani; Sindhu, R.; Singh, I. Phytosterols: Physiological functions and therapeutic applications. In Bioactive Food Components Activity in Mechanistic Approach; Cazarin, C.B.B., Pastore, G.M., Bicas, J.L., Morostica, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 223–238. [Google Scholar]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Adelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Paduch, R.; Kandefer-Szerszeń, M. Antitumor and antiviral activity of pentacyclic triterpenes. Mini-Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandeep; Ghosh, S. Triterpenoids: Structural diversity, biosynthetic pathways and bioactivity. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 411–461. [Google Scholar]
- Tolufashe, G.F.; Lawal, M.M.; Govender, K.K.; Shode, F.O.; Singh, T. Exploring the bioactivity of pentacyclic triterpenoids as potential antimycobacterial nutraceutics: Insights through comparative biomolecular modeling. J. Mol. Graph. Model. 2021, 105, 1007900. [Google Scholar] [CrossRef]
- Harley, B.K.; Neglo, D.; Tawiah, P.; Pipim, M.A.; Mireku-Gyimah, N.A.; Tettey, C.O.; Amengor, C.D.; Fleisher, T.C.; Waikhom, S.D. Bioactive triterpenoids from Solanum torvum fruits with antifungal, resistance modulatory and antibiofilm formation activities against fluconazole-resistant Candida albicans strains. PLoS ONE 2021, 16, e0260956. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Maccioni, D.; Sanjust, E.; Falconieri, D.; Farris, E.; Maxia, A. Ethnopharmacobotany and diversity of Mediterranean endemic plants in Marmilla subregion, Sardinia, Italy. Plants 2022, 11, 3165. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, D.; Papanikolaou, A.; Falaza, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring lab dane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Alaoui Ismaili, S.; Zair, T. Chemical composition, antibacterial and antifungal activity of the essential oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef]
- Sayah, K.; Marmouzi, I.; Mrabti, H.N.; Cherrah, Y.; El Abbes Faouzi, M. Antioxidant activity and inhibitory potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) aerial parts extracts against key enzymes linked to hyperglycemia. BioMed Res. Intl. 2017, 2017, 2789482. [Google Scholar] [CrossRef] [Green Version]
- Haida, S.; Bakkouche, K.; Kribii, A.R.; Kribii, A. Chemical composition of essential oil, phenolic compounds, and antioxidant activity of Cistus monspeliensis from Northern Morocco. Biochem. Res. Int. 2021, 2021, 6669877. [Google Scholar] [CrossRef]
- Dias, P.; Falé, P.L.; Martins, A.; Rauter, A.P. Digestibility and bioavailability of the active components of Erica australis L. aqueous extracts and their therapeutic potential as acetylcholinesterase inhibitors. Evid.-Based Complement. Altern. Med. 2015, 2015, 854373. [Google Scholar] [CrossRef] [Green Version]
- Nazemiyeh, H.; Bahadori, F.; Delazar, A.; Ay, M.; Topçu, G.; Nahar, L.; Majinda, R.R.T.; Sarker, S.D. Antioxidant phenolic compounds from the leaves of Erica arborea (Ericaceae). Nat. Prod. Res. 2008, 22, 1385–1392. [Google Scholar] [CrossRef]
- Bessah, R.; Benyoussef, E.-H. Essential oil composition of Erica arborea L. leaves from Algeria. J. Essent. Oil-Bear. Plants 2014, 17, 931–935. [Google Scholar] [CrossRef]
- Khlifi, D.; Hamdi, M.; El Hayouni, A.; Cazaux, S.; Souchard, J.-P.; Couderc, F.; Bouajila, J. Global chemical composition and antioxidant and anti-tuberculosis activities of various extracts of Globularia alypum L. (Globulariaceae) leaves. Molecules 2011, 16, 10592–10603. [Google Scholar] [CrossRef] [PubMed]
- Asraoui, F.; Kounnoun, A.; Cadi, H.E.; Cacciola, F.; Majdoub, Y.O.E.; Alibrando, F.; Mandolfino, F.; Dugo, P.; Mondello, L.; Louajri, A. Phytochemical investigation and antioxidant activity of Globularia alypum L. Molecules 2021, 26, 759. [Google Scholar] [CrossRef]
- Friščić, M.; Petlevski, R.; Kosalec, I.; Madunić, J.; Matulić, M.; Bucar, F.; Hazler Pilepić, K.; Maleš, Ž. Globularia alypum L. and related species: LC-MS profiles and antidiabetic, antioxidant, anti-inflammatory, antibacterial and anticancer potential. Pharmaceuticals 2022, 15, 506. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Surmaghi, M.H.S.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [Green Version]
- Ghzaiel, I.; Zarrouk, A.; Nury, T.; Libergoli, M.; Florio, F.; Hammouda, S.; Ménétrier, F.; Avoscan, L.; Yammine, A.; Samadi, M.; et al. Antioxidant properties and cytoprotective effect of Pistacia lentiscus L. seed oil against 7β-hydroxycholesterol-induced toxicity in C2C12 myoblasts: Reduction in oxidative stress, mitochondrial and peroxisomal dysfunctions and attenuation of cell death. Antioxidants 2021, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Nekkaa, A.; Benaissa, A.; Mutelet, F.; Canabady-Rochelle, L. Rhamnus alaternus plant: Extraction of bioactive fractions and evaluation of their pharmacological and phytochemical properties. Antioxidants 2021, 10, 300. [Google Scholar] [CrossRef]
- Gadouche, L.; Zerrouki, K.; Zidane, A.; Ababou, A.; Bachir Elazaar, I.; Merabet, D.; Henniche, W.; Ikhlef, S. Genoprotective, antimutagenic, and antioxidant effect of methanolic leaf extract of Rhamnus alaternus L. from the Bissa mountains in Algeria. Foods Raw Mater. 2022, 10, 196–205. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Huttunen, S. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef]
- Dos Santos, V.A.; Dos Santos, D.P.; Castro-Gamboa, I.; Zanoni, M.V.; Furlan, M. Evaluation of antioxidant capacity and synergistic associations of quinonemethide triterpenes and phenolic substances from Maytenus ilicifolia (Celastraceae). Molecules 2010, 15, 6956–6973. [Google Scholar] [PubMed] [Green Version]
- Rogowska, A.; Pączkowski, C.; Szakiel, A. Modulation of steroid and triterpenoid metabolism in Calendula officinalis plants and hairy root cultures exposed to cadmium stress. Int. J. Mol. Sci. 2022, 23, 5640. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Stpiczyńska, M.; Pączkowski, C.; Szakiel, A. The influence of exogenous jasmonic acid on the biosynthesis of steroids and triterpenoids in Calendula officinalis plants and hairy root culture. Int. J. Mol. Sci. 2022, 23, 12173. [Google Scholar] [CrossRef] [PubMed]
- Masa, C.V.; Gallego, J.C.A.; Lobón, N.C.; Díaz, T.S. Intra-population variation of secondary metabolites in Cistus ladanifer L. Molecules 2016, 21, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szakiel, A.; Grabarczyk, M.; Pączkowski, C.; Mieczkowski, A. Comparison of the profiles of non-glycosylated triterpenoids from leaves of plants of selected species of genus Dioscorea. Phyt. Lett. 2017, 20, 350–355. [Google Scholar] [CrossRef]
- Rogowska, A.; Styczyński, M.; Pączkowski, C.; Szakiel, A.; Pinheiro de Carvalho, M.Â.A. GC-MS analysis of steroids and triterpenoids occurring in leaves and tubers of Tamus edulis Lowe. Phyt. Lett. 2019, 30, 231–234. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Becker, R.; Pączkowski, C.; Szakiel, A. Various patterns of composition and accumulation of steroids and triterpenoids in cuticular waxes from screened Ericaceae and Caprifoliaceae berries during fruit development. Molecules 2019, 24, 3826. [Google Scholar] [CrossRef] [Green Version]
- Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Variations in triterpenoid deposition in cuticular waxes during development and maturation of selected fruits of Rosaceae family. Int. J. Mol. Sci. 2020, 21, 9762. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Szakiel, A.; Niżyński, B.; Pączkowski, C. Triterpenoid profile of flower and leaf cuticular waxes of heather Calluna vulgaris. Nat. Prod. Res. 2013, 27, 1404–1407. [Google Scholar] [CrossRef]
- Shangmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpeoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Ghonte, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: An overview on targets and underlying mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.; Shalini; Verma, K.; Kumar, I. A rewiew on pharmacological activities of lupeol and its triterpene derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar] [CrossRef]
- Boutemak, K.; Safta, B.; Ayachi, N. Study of the antiinflammatory activity of flavonic extract of Globularia alypum L. Acta Phys. Pol. A 2015, 128, 239–240. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. 2018. Long-Term consumption of cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy Korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Harrabi, S.; Ferchichi, A.; Bacheli, A.; Fellah, H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybum marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018, 17, 82. [Google Scholar] [CrossRef] [Green Version]
- Hadjimbei, E.; Botsaris, G.; Goulas, V.; Gekas, V. Health-promoting effects of Pistacia resins: Recent advances, challenges, and potential applications in the food industry. Food Rev. Int. 2015, 31, 1–12. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Feng, Y.-P.; Liu, W.; Yuan, T. Diverse triterpenoids from mastic produced by Pistacia lentiscus and their antiinflammtory activities. Chem. Biodivers. 2022, 19, e202101012. [Google Scholar]
- Liu, W.; Gao, J.; Li, M.; Aisa, H.A.; Yuan, T. Tirucallane triterpenoids from the mastic (Pistacia lentiscus) and their anti-inflammatory and cytotoxic activities. Phytochemistry 2022, 182, 112596. [Google Scholar] [CrossRef]
- An, X.; Wang, J.; Yu, X.; Wu, H.; Liu, W. Two new polypodane-type bicyclic triterpenoids from mastic. Open Chem. 2022, 20, 267–271. [Google Scholar] [CrossRef]
- Trabelsi, H.; Sakouhi, F.; Renaud, J.; Villeneuve, P.; Khouja, M.L.; Mayer, P.; Boukhchina, S. Fatty acids, 4-desmethylsterols, and triterpene alcohols from Tunisian lentisc (Pistacia lentiscus) fruits. Eur. J. Lipid Sci. Technol. 2012, 114, 968–973. [Google Scholar] [CrossRef]
- Qabaha, K.; Ras, S.A.; Abbadi, J.; Al-Rimawi, F. Anti-inflammatory activity of Eucalyptus spp. and Pistacia lentiscus leaf extracts. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Chen, G.; Li, V.; Soler, F.; Guo, M. Analysis of flavonoids in Rhamnus davrica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, L.H.; Palazon, J.; Navarro-Ocańa, A. The pentacyclic triterpenes α, β-amyrins: A review of sources and biological activities. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; In-Tech Open: London, UK, 2022; pp. 487–502. [Google Scholar]
- Guefack, M.-G.F.; Ngangoue, M.O.; Mbaveng, A.T.; Nayim, P.; Kuete, J.R.N.; Carine Ngafo, M.N.; Chi, G.F.; Ngameni, B.; Ngadjui, B.T.; Kuete, V. Antibacterial and antibiotic-potentiation activity of the constituents from aerial part of Donella welwitshii (Sapotaceae) against multidrug resistant phenotypes. BMC Complement. Med. Ther. 2022, 22, 194. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its component. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Zhang, R. Purification and antioxidant properties of triterpenoid acids from blackened jujube (Ziciphus jujuba Mill.) by macroporous resin. Food Sci. Nutr. 2021, 9, 5070–5092. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Lin, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ababou, A.; Chouieb, M.; Saidi, D.; Bouthiba, A.; Mederbal, K. Analyse statistique de la diversité floristique dans la région de Beni-Haoua, Chlef, Algérie. NATEC 2017, 9, 16–22. [Google Scholar]
- Quezel, P.; Santa, S. New Flora of Algeria and Southern Desert Regions; National Centre for Scientific Research: Paris, France, 1963; Volume 2, p. 1170. [Google Scholar]
- Szakiel, A.; Voutquenne-Nazabadioko, L.; Henry, M. Isolation and biological activities of lyoniside from rhizomes and stems of Vaccinium myrtillus. Phyt. Lett. 2011, 4, 138–143. [Google Scholar] [CrossRef]




| Compounds/Category | Cistus ladanifer | Cistus monspeliensis |
|---|---|---|
| Content [µg/g d.w. ± SD] | ||
| sterols: | ||
| campesterol | 40.24 ± 2.23 a | 40.59 ± 2.55 a |
| sitosterol | 571.97 ± 30.06 a | 490.95 ±12.66 b |
| stigmasterol | 22.60 ± 1.25 a | 32.50 ± 2.46 b |
| sum of sterols | 634.81 | 564.04 |
| steroid ketones: | ||
| sitostenone | 24.75 ± 1.60 a | 27.29 ± 1.69 a |
| stigmasta-3,6-dione | 46.81 ± 1.81 a | 58.64 ± 2.37 b |
| tremulone | 22.51 ± 1.40 a | 16.89 ± 1.64 b |
| sum of steroid ketones | 94.07 | 102.82 |
| sum of steroids | 728.88 | 666.86 |
| neutral triterpenoids: | ||
| α-amyrin | 280.80 ± 10.79 a | 419.30 ± 20.71 b |
| β-amyrin | 120.69 ± 3.33 a | 370.40 ± 12.47 b |
| sum of neutral triterpenoids | 401.49 | 789.70 |
| triterpenoid acids: | ||
| maslinic acid | 31.82 ± 1.98 a | 337.24 ± 15.98 b |
| maslinic acid methyl ester | n.d. | 50.03 ± 2.49 a |
| oleanolic acid | 34.61 ± 2.49 a | 79.50 ± 11.48 b |
| 3-oxooleanolic acid | 19.10 ± 1.21 a | 93.46 ± 13.62 b |
| ursolic acid | 60.27 ± 2.58 a | 8.49 ± 0.54 b |
| sum of acids | 145.80 | 568.72 |
| sum of triterpenoids | 547.29 | 1358.42 |
| Total | 1276.17 | 2025.28 |
| Compounds/Category | Content [µg/g d.w. ± SD] |
|---|---|
| sterols: | |
| campesterol | 304.60 ± 10.52 b |
| sitosterol | 846.15 ± 16.37 c |
| sum of sterols | 1150.75 |
| steroid ketones: | |
| sitostenone | 50.49 ± 2.51 b |
| tremulone | 82.73 ± 6.09 c |
| sum of ketones | 133.22 |
| sum of steroids | 1283.97 |
| neutral triterpenoids: | |
| α-amyrin/lupeol | 23,809.86 ± 322.07 c |
| α-amyrenone/lupenone | 502.10 ± 17.23 a |
| β-amyrin | 2396.95 ± 87.87 c |
| β-amyrenone | 618.19 ± 14.43 a |
| taraxasterol | 75.52 ± 3.53 a |
| oleanolic aldehyde | 74.46 ± 2.35 a |
| ursolic aldehyde | 615.07 ± 18.70 a |
| betulin | 204.78 ± 13.60 a |
| erythrodiol | 38.53 ± 1.86 a |
| uvaol | 158.09 ± 14.37 a |
| sum of neutral triterpenoids | 28,493.55 |
| triterpenoid acids: | |
| betulinic acid | 573.12 ± 13.18 a |
| maslinic acid | 641.61 ± 12.97 c |
| oleanolic acid | 6022.89 ± 72.67 c |
| 3-oxooleanolic acid | 205.30 ± 15.97 c |
| olean-2,12-dien-28-oic acid | 133.11 ± 7.15 a |
| ursolic acid | 14,889.49 ± 129.99 c |
| 3-oxoursolic acid | 3570.32 ± 56.77 c |
| ursa-2,12-dien-28-oic acid | 1007.38 ± 25.27 a |
| sum of acids | 27,043.22 |
| sum of triterpenoids | 55,536.77 |
| Total | 56,820.74 |
| Compounds/Category | Content [µg/g d.w. ± SD] |
|---|---|
| sterols: | |
| campesterol | 24.63 ± 2.25 c |
| sitosterol | 436.94 ± 20.46 d |
| stigmasterol | 17.94 ± 2.09 c |
| sum of sterols | 479.51 |
| steroid ketones: | |
| sitostenone | 62.61 ± 4.44 c |
| stigmasta-3,6-dione | 95.31 ± 12.83 c |
| tremulone | 53.34 ± 4.10 d |
| sum of steroid ketones | 211.26 |
| sum of steroids | 690.77 |
| neutral triterpenoids: | |
| α-amyrin/lupeol | 52.06 ± 3.90 d |
| α-amyrenone/lupenone | 26.77 ± 2.14 b |
| β-amyrin | 36.35 ± 2.29 d |
| betulin | 73.69 ± 10.64 b |
| sum of neutral triterpenoids | 188.87 |
| triterpenoid acids: | |
| oleanolic acid | 47.98 ±3.87 d |
| ursolic acid | 74.95 ± 5.36 d |
| sum of acids | 122.55 |
| sum of triterpenoids | 311.42 |
| Total | 1002.19 |
| Compounds/Category | Content [µg/g d.w. ± SD] |
|---|---|
| sterols: | |
| campesterol | 39.43 ± 2.18 a |
| sitosterol | 1356.62 ± 60.63 e |
| stigmasterol | 32.35 ± 2.57 b |
| sum of sterols | 1428.4 |
| steroid ketones: | |
| sitostenone | 126.75 ± 15.16 d |
| tremulone | 37.48 ± 2.23 d |
| sum of ketones | 164.23 |
| sum of steroids | 1592.63 |
| neutral triterpenoids: | |
| α-amyrin/lupeol | 5121.09 ± 125.03 e |
| α-amyrenone/lupenone | 606.64 ± 27.36 c |
| β-amyrin | 307.68 ± 22.7 a |
| lupeol acetate | 31.89 ± 3.36 a |
| oleanolic aldehyde | 82.79 ± 11.11 a |
| ursolic aldehyde | 96.57 ± 7.06 b |
| erythrodiol | 28.41 ± 3.36 b |
| uvaol | 33.26 ± 3.00 b |
| betulin | 4.97 ± 0.14 c |
| sum of neutral triterpenoids | 6313.3 |
| triterpenoid acids: | |
| morolic acid | 272.12 ± 14.17 a |
| moronic acid | 118.27 ± 17.30 a |
| oleanolic acid | 357.09 ± 17.39 b |
| 3-oxooleanolic acid | 3222.65 ± 74.40 d |
| sum of acids | 3970.13 |
| sum of triterpenoids | 10,283.43 |
| Total | 11,876.06 |
| Compounds/Category | Content [µg/g d.w. ± SD] |
|---|---|
| sterols | |
| campesterol | 59.92 ± 3.79 d |
| sitosterol | 309.10 ± 12.87 f |
| stigmasterol | 66.49 ± 4.43 d |
| sum of sterols | 435.51 |
| steroid ketones: | |
| sitostenone | 36.72 ± 1.88 e |
| stigmasta-3,6-dione | 85.81 ± 5.55 d |
| tremulone | 29.73 ± 1.69 a |
| sum of ketones | 152.26 |
| sum of steroids | 587.77 |
| triterpenoids: | |
| α-amyrin/lupeol | 533.31 ± 20.86 b |
| β-amyrin | 415.07 ± 25.32 b |
| germanicol | 382.16 ± 20.73 a |
| oleanolic aldehyde | 113.47 ± 11.80 b |
| ursolic aldehyde | 203.64 ± 23.46 c |
| sum of triterpenoids | 1647.65 |
| Total | 2235.42 |
| Extract | Radical Scavenging Activity (%) |
|---|---|
| Cistus ladanifer | 27.8 ± 1.20 |
| Cistus monspelliensis | 69.9 ± 2.04 |
| Erica arborea | 77.3 ± 2.86 |
| Globularia alypum | 84.1 ± 3.05 |
| Pistacia lentiscus | 64.7 ± 2.12 |
| Rhamnus alaternus | 56.7 ± 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadouche, L.; Alsoufi, A.S.M.; Pacholska, D.; Skotarek, A.; Pączkowski, C.; Szakiel, A. Triterpenoid and Steroid Content of Lipophilic Extracts of Selected Medicinal Plants of the Mediterranean Region. Molecules 2023, 28, 697. https://doi.org/10.3390/molecules28020697
Gadouche L, Alsoufi ASM, Pacholska D, Skotarek A, Pączkowski C, Szakiel A. Triterpenoid and Steroid Content of Lipophilic Extracts of Selected Medicinal Plants of the Mediterranean Region. Molecules. 2023; 28(2):697. https://doi.org/10.3390/molecules28020697
Chicago/Turabian StyleGadouche, Leila, Abdulwadood Shakir Mahmood Alsoufi, Dominika Pacholska, Anna Skotarek, Cezary Pączkowski, and Anna Szakiel. 2023. "Triterpenoid and Steroid Content of Lipophilic Extracts of Selected Medicinal Plants of the Mediterranean Region" Molecules 28, no. 2: 697. https://doi.org/10.3390/molecules28020697
APA StyleGadouche, L., Alsoufi, A. S. M., Pacholska, D., Skotarek, A., Pączkowski, C., & Szakiel, A. (2023). Triterpenoid and Steroid Content of Lipophilic Extracts of Selected Medicinal Plants of the Mediterranean Region. Molecules, 28(2), 697. https://doi.org/10.3390/molecules28020697