Structure, Optical and Magnetic Properties of Two Isomeric 2-Bromomethylpyridine Cu(II) Complexes [Cu(C6H9NBr)2(NO3)2] with Very Different Binding Motives
Abstract
:1. Introduction
2. Results and Discussion
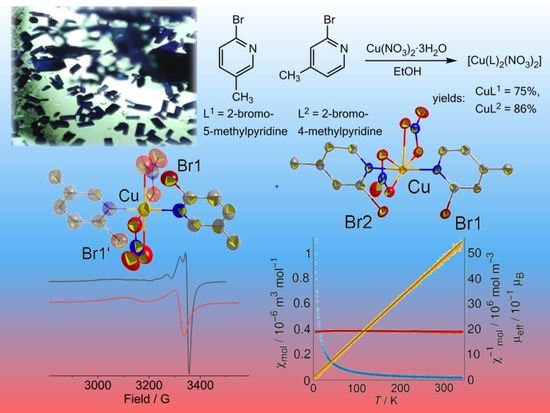
2.1. Syntheses and Analyses
2.2. Crystal Structures
2.3. Hirshfeld Surface Analysis and Energy Framework Calculations
2.4. Infrared and Raman Spectroscopy
2.5. Thermal Analysis
2.6. UV–Vis Absorption Spectroscopy
2.7. Magnetization and EPR Studies
3. Experimental Section
3.1. Chemicals
3.2. Syntheses
3.2.1. Synthesis of [Cu(L1)2(NO3)2] L1 = 2-Bromo-5-methylpyridine
3.2.2. Synthesis of [Cu(L2)2(NO3)2] L2 = 2-Bromo-4-methylpyridine
3.3. Instrumentation
3.4. Magnetization Measurements
3.5. Single-Crystal X-ray Diffraction
3.6. Powder X-ray Diffraction
3.7. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- D’Vries, R.F.; Gomez, G.E.; Ellena, J. Highlighting Recent Crystalline Engineering Aspects of Luminescent Coordination Polymers Based on F-Elements and Ditopic Aliphatic Ligands. Molecules 2022, 27, 3830. [Google Scholar] [CrossRef] [PubMed]
- Ashbridge, Z.; Fielden, S.D.P.; Leigh, D.A.; Pirvu, L.; Schaufelberger, F.; Zhang, L. Knotting matters: Orderly molecular entanglements. Chem. Soc. Rev. 2022, 51, 7779–7809. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Constable, E.C. The terpyridine isomer game: From chelate to coordination network building block. Chem. Commun. 2020, 56, 10786–10794. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, J. Heterometallic grids: Synthetic strategies and recent advances. Dalton Trans. 2019, 48, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Fugu, M.B.; Ellaby, R.J.; O’Connor, H.M.; Pitak, M.B.; Klooster, W.; Horton, P.N.; Coles, S.J.; Al-Mashhadani, M.H.; Perepichka, I.F.; Brechin, E.K.; et al. Mono- and ditopic hydroxamate ligands towards discrete and extended network architectures. Dalton Trans. 2019, 48, 10180–10190. [Google Scholar] [CrossRef]
- Shiga, T.; Newton, G.N.; Oshio, H. Pre-programmed self-assembly of polynuclear clusters. Dalton Trans. 2018, 47, 7384–7394. [Google Scholar] [CrossRef]
- Ahmad, N.R.; Chughtai, A.H.; Younus, H.A.; Verpoort, F. Discrete metal-carboxylate self-assembled cages: Design, synthesis and applications. Coord. Chem. Rev. 2014, 280, 1–27. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal-Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [Green Version]
- Dawe, L.N.; Shuvaev, K.V.; Thompson, L.K. Polytopic ligand directed self-assembly—Polymetallic [n × n] grids versus non-grid oligomers. Chem. Soc. Rev. 2009, 38, 2334–2359. [Google Scholar] [CrossRef]
- Guillerm, V.; Eddaoudi, M. The Importance of Highly Connected Building Units in Reticular Chemistry: Thoughtful Design of Metal-Organic Frameworks. Acc. Chem. Res. 2021, 54, 3298–3312. [Google Scholar] [CrossRef]
- Fernández-Figueiras, A.; Ravutsov, M.A.; Simeonov, S.P. Site-Selective C–H Functionalization of Arenes Enabled by Noncovalent Interactions. ACS Omega 2022, 8, 6439–6448. [Google Scholar] [CrossRef]
- Lohith, T.N.; Hema, M.K.; Karthik, C.S.; Sandeep, S.; Mallesha, L.; Mallu, P.; Ramalingam, R.J.; Sridhar, M.A.; Karnan, M.; Lokanath, N.K. N-[2-(5-bromo-2-chloro-pyrimidin-4-yl)thio)-4-methoxy-phenyl]-4-chlorobenzenesulfonamide: The existence of H-bond and halogen bond interactions assisted supramolecular architecture—A quantum chemical investigation. J. Mol. Struct. 2022, 1267, 133476. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-Covalent Intramolecular Interactions through Ligand-Design Promoting Efficient Luminescence from Transition Metal Complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, H.; Yagishita, S.; Kanazashi, K.; Ishii, M.; Hayami, S.; Konaka, H.; Ishikawa, R.; Kawata, S. Hydrogen-Bonding Assembly of Coordination Polymers Showing Reversible Dynamic Solid-State Structural Transformations. Inorganics 2018, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Siu, S.K.-L.; Po, C.; Yim, K.-C.; Au, V.K.-M.; Yam, V.W.-W. Synthesis, characterization and spectroscopic studies of luminescent L-valine modified alkynyl-based cyclometalated gold(III) complexes with gelation properties driven by π–π stacking, hydrogen bonding and hydrophobic–hydrophobic interactions. Cryst. Eng. Comm. 2015, 17, 8153–8162. [Google Scholar] [CrossRef]
- Wang, R.; Dols, T.S.; Lehmann, C.W.; Englert, U. The halogen bond made visible: Experimental charge density of a very short intermolecular Cl⋯Cl donor–acceptor contact. Chem. Commun. 2012, 48, 6830–6832. [Google Scholar] [CrossRef]
- Biradha, K.; Goswami, A.; Moi, R. Coordination polymers as heterogeneous catalysts in hydrogen evolution and oxygen evolution reactions. Chem. Commun. 2020, 56, 10824–10842. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Q.; Ni, Y.; Shang, L.; Zhang, X.; Yan, Z.; Zhao, Q.; Chen, J. Rational design and synthesis of two-dimensional conjugated metal-organic polymers for electrocatalysis applications. Chem 2022, 8, 1822–1854. [Google Scholar] [CrossRef]
- Pachisia, S.; Gupta, R. Architectural and catalytic aspects of designer materials built using metalloligands of pyridine-2,6-dicarboxamide based ligands. Dalton Trans. 2020, 49, 14731–14748. [Google Scholar] [CrossRef]
- Kuwamura, N.; Konno, T. Heterometallic coordination polymers as heterogeneous electrocatalysts. Inorg. Chem. Front. 2021, 8, 2634. [Google Scholar] [CrossRef]
- Liu, Q.-Q.; Wang, X.-L.; Lin, H.-Y.; Chang, Z.-H.; Zhang, Y.-C.; Tian, Y.; Lu, J.-J.; Yu, L. Two new polyoxometalate-based metal–organic complexes for the detection of trace Cr(VI) and their capacitor performance. Dalton Trans. 2021, 50, 9450–9456. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Panda, S.K.; Singh, A.K. Recent developments in supramolecular complexes of azabenzenes containing one to four N atoms: Synthetic strategies, structures, and magnetic properties. RSC Adv. 2022, 12, 18945–18972. [Google Scholar] [CrossRef] [PubMed]
- Mylonas-Margaritis, I.; Gérard, A.; Skordi, K.; Mayans, J.; Tasiopoulos, A.; McArdle, P.; Papatriantafyllopoulou, C. From 1D Coordination Polymers to Metal Organic Frameworks by the Use of 2-Pyridyl Oximes. Materials 2020, 13, 4084. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Novikov, A.S.; Chernova, K.V.; Vinnik, D.A.; Taskaev, S.V.; Korolkov, I.V.; Ilyina, E.V.; Pavlov, A.A.; Novikov, V.V.; Sokolov, M.N.; et al. Heteroleptic copper(II) complexes with 2-bromo-5-methylpyridine: Structures, features of non-covalent interactions and magnetic behavior. Inorg. Chim. Acta 2020, 502, 119333. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Zhang, G.-D.; Sheng, X.-X.; Ding, Q.-W.; Bai, Y.-Z.; Su, Y.; Liu, H.-K.; Su, Z. Efficient MO Dye Degradation Catalyst of Cu(I)-Based Coordination Complex from Dissolution-Recrystallization Structural Transformation. Cryst. Growth Des. 2021, 21, 333–343. [Google Scholar] [CrossRef]
- Chen, X.; Yang, F.; Han, C.; Han, L.; Wang, F.; Jin, G.; Wang, H.; Ma, J. [Fe2S2−Agx]-Hydrogenase Active-Site-Containing Coordination Polymers and Their Photocatalytic H2 Evolution Reaction Properties. Inorg. Chem. 2022, 61, 13261–13265. [Google Scholar] [CrossRef]
- Hornberger, L.-S.; Adams, F. Photocatalytic CO2 Conversion Using Metal-Containing Coordination Polymers and Networks: Recent Developments in Material Design and Mechanistic Details. Polymers 2022, 14, 2778. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Calvet, T.; Font-Bardia, M.; Domingo, C.; Ayllón, J.A.; Pons, J. Modulating p-hydroxycinnamate behavior as a ditopic linker or photoacid in copper(II) complexes with an auxiliary pyridine ligand. Dalton Trans. 2018, 47, 6479–6493. [Google Scholar] [CrossRef] [Green Version]
- Vittal, J.J.; Quah, H.S. Photochemical reactions of metal complexes in the solid state. Dalton Trans. 2017, 46, 7120–7140. [Google Scholar] [CrossRef]
- Garai, M.; Biradha, K. One-Dimensional Coordination Polymers of Bis(3-pyridylacrylamido)ethane: Influence of Anions and Metal Ions on Their Solid State [2 + 2] Photochemical Polymerization and Dimerization Reactions. Cryst. Growth Des. 2017, 17, 925–932. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Zhang, M.; Pei, R.-B.; Wang, Q.; Wei, D.-H.; Zang, S.-Q.; Fan, Y.-T.; Mak, T.C.W. A Crystalline Copper(II) Coordination Polymer for the Efficient Visible-Light-Driven Generation of Hydrogen. Angew. Chem. Int. Ed. 2016, 55, 2073–2077. [Google Scholar] [CrossRef]
- Dai, M.; Li, H.-X.; Lang, J.-P. New approaches to the degradation of organic dyes, and nitro- and chloroaromatics using coordination polymers as photocatalysts. Cryst. Eng. Comm. 2015, 17, 4741–4753. [Google Scholar] [CrossRef]
- Gorai, T.; Schmitt, W.; Gunnlaugsson, T. Highlights of the development and application of luminescent lanthanide based coordination polymers, MOFs and functional nanomaterials. Dalton Trans. 2021, 50, 770–784. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Tsurui, M.; Hasegawa, Y. Steric and Electronic Control of Chiral Eu(III) Complexes for Effective Circularly Polarized Luminescence. ACS Omega 2020, 5, 3786–3791. [Google Scholar] [CrossRef] [Green Version]
- Yue, Q.; Gao, E.-Q. Azide and carboxylate as simultaneous coupler for magnetic coordination polymers. Coord. Chem. Rev. 2019, 382, 1–31. [Google Scholar] [CrossRef]
- Castro, I.; Barros, W.P.; Calatayud, M.L.; Lloret, F.; Marino, N.; De Munno, G.; Stumpf, H.O.; Ruiz-García, R.; Julve, M. Dicopper(II) pyrazolenophanes: Ligand effects on their structures and magnetic properties. Coord. Chem. Rev. 2016, 315, 135–152. [Google Scholar] [CrossRef]
- Roy, M.; Adhikary, A.; Debnath, T.; Das, A.K.; Mondal, R. Designing ferromagnetism in Cu(II) complexes using an elusive near-orthogonal bridging mode of the pyrazole ring. Polyhedron 2019, 160, 46–52. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Y.-Q.; Li, J.; Gao, Q. A Cu(II) coordination framework constructed by the inorganic layer and a bent dipyridyl ligand: Synthesis, structure and magnetic properties. J. Ind. Chem. Soc. 2021, 98, 100125. [Google Scholar] [CrossRef]
- Li, S.-X.; Qiang, J.-W.; Liao, B.-L. Structure, magnetism and oxygen reduction reaction in mixed-valent Cu(I)⋯Cu(II) complex supported by benzimidazole derivative. Inorg. Chim. Acta 2021, 521, 120356. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; He, M.-Q.; Li, M.-X.; Huang, Y.-Q.; Chi, T.; Wang, Z.-X. Ferrimagnetic copper-carboxyphosphinate compounds for catalytic degradation of methylene blue. Inorg. Chem. Commun. 2018, 94, 5–9. [Google Scholar] [CrossRef]
- Chakraborty, T.; Sarkar, A.; Adhikary, A.; Chakiroy, N.; Das, D. Synthesis of Structurally Diverse Ferrimagnetically and Antiferromagnetically Coupled MII−MnII (M = Cu, Ni) Heterometallic Schiff Base Compounds with a Dicyanamide Spacer and Study of Biomimetic Catalytic Activity. Cryst. Growth Des. 2019, 19, 7336–7348. [Google Scholar] [CrossRef]
- Zordan, F.; Brammer, L. M−X···X‘−C Halogen-Bonded Network Formation in MX2(4-halopyridine)2 Complexes (M = Pd, Pt; X = Cl, I.; X‘ = Cl, Br, I). Cryst. Growth Des. 2006, 6, 1374–1379. [Google Scholar] [CrossRef]
- Nicholas, A.D.; Otten, B.M.; Ayala, G.; Hutchinson, J.; Wojtas, L.; Omary, M.A.; Pike, R.D.; Patterson, H.H. Light-Induced Photochemical Changes in Copper(I) Thiocyanate Complexes Decorated with Halopyridines: Optical Memory Manifestation. J. Phys. Chem. C 2017, 121, 25430–25439. [Google Scholar] [CrossRef] [Green Version]
- Vitorica-Yrezabal, I.J.; Sullivan, R.A.; Purver, S.L.; Curfs, C.; Tang, C.C.; Brammer, L. Synthesis and polymorphism of (4-ClpyH)2[CuCl4]: Solid–gas and solid–solid reactions. Cryst. Eng. Comm. 2011, 13, 3189–3196. [Google Scholar] [CrossRef]
- Wackerbarth, I.; Widhyadnyani, N.N.A.T.; Schmitz, S.; Stirnat, K.; Butsch, K.; Pantenburg, I.; Meyer, G.; Klein, A. CuII Complexes and Coordination Polymers with Pyridine or Pyrazine Amides and Amino Benzamides—Structures and EPR Patterns. Inorganics 2020, 8, 65. [Google Scholar] [CrossRef]
- Halcrow, M.A. Jahn–Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem. Soc. Rev. 2013, 42, 1784–1795. [Google Scholar] [CrossRef] [Green Version]
- Kilner, C.A.; McInnes, E.J.L.; Leech, M.A.; Beddard, G.S.; Howard, J.A.K.; Mabbs, F.E.; Collison, D.; Bridgeman, A.J.; Halcrow, M.A. A crystallographic, EPR and theoretical study of the Jahn–Teller distortion in [CuTp2] (Tp− = tris{pyrazol-1-yl}hydridoborate). Dalton Trans. 2004, 2, 236–243. [Google Scholar] [CrossRef]
- Xuan, R.; Li, M.; Wan, Y. Bis(2-chloro-5-methylpyridine-κN)-bis(nitrato-κ2O,O′)copper(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, C59, m462–m464. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.-B.; Shan, R.-Y.; Yang, W.-N.; Gou, S.-H. From Zero-dimensional to One-dimensional: Use of Metal-Ligand Affinity in Supramolecular Assembly. Z. Anorg. Allg. Chem. 2013, 639, 125–128. [Google Scholar] [CrossRef]
- Puszko, A.; Krojcer, A.; Pełczynska, M.; Wietrzyk, J.; Cieslak-Golonka, M.; Jezierska, J.; Adach, A.; Kubiak, M. Mononuclear copper(II) nitrato complexes with methyl-substituted 4-nitropyridine N-oxide. Physicochemical and cytotoxic characteristics. J. Inorg. Biochem. 2010, 104, 153–160. [Google Scholar] [CrossRef]
- Cameron, A.F.; Forrest, K.P.; Taylor, D.W.; Nuttall, R.H. Structural Investigations of Metal Nitrate Complexes. Part, I. Crystal and Molecular Structure of Bis[dinitratobis(pyridine)copper(II)]-Pyridine [Cu(NO3)2(py)2]2. Py. J. Chem. Soc. A 1971, 2492–2496. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [Green Version]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Dey, D.; Bhandary, S.; Thomas, S.P.; Spackman, M.A.; Chopra, D. Energy frameworks and a topological analysis of the supramolecular features in in situ cryocrystallized liquids: Tuning the weak interaction landscape via fluorination. Phys. Chem. Chem. Phys. 2016, 18, 31811–31820. [Google Scholar] [CrossRef] [Green Version]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2001; ISBN 9780470093078. [Google Scholar]
- Arjunan, V.; Senthilkumari, S.; Ravindran, P.; Mohan, S. Synthesis, FTIR and FT-Raman spectral analysis and structure–activity relations of N-(4-bromophenyl)-2,2-dichloroacetamide by DFT studies. J. Mol. Struct. 2014, 1064, 15–26. [Google Scholar] [CrossRef]
- Sambathkumar, K. Analysis of vibrational Spectra of 2-Amino-5-Bromo-4-Methylpyridine Based on Ab Initio and Density Functional Theory Calculations. Elixir Vib. Spec. 2016, 91, 38381–38391. [Google Scholar]
- Warad, I.; Musameh, S.; Badran, I.; Nassar, N.N.; Brandao, P.; Tavares, C.J.; Barakat, A. Synthesis, solvatochromism and crystal structure of trans-[Cu(Et2NCH2CH2NH2)2(H2O)](NO3)2 complex: Experimental with DFT combination. J. Mol. Struct. 2017, 1148, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Harzi, F.; Arfaoui, Y.; Silvestru, C.; Bourguiba, N.F. Synthesis, structural and spectroscopic studies, DFT calculations, thermal characterization and Hirshfeld surface analysis of copper(II) organic-inorganic hybrid material (C12H22N2)[CuCl4]. J. Coord. Chem. 2021, 75, 70–83. [Google Scholar] [CrossRef]
- Xu, G.C.; Zhang, L.; Liu, L.; Liu, G.F.; Jia, D.Z. Thermal kinetic TG-analysis of the mixed-ligand copper(II) and nickel(II) complexes of N-(1-phenyl-3-methyl-4-benzylidene-5-pyrazolone) p-nitrobezoylhydrazide and pyridine. Thermochim. Acta 2005, 429, 31–42. [Google Scholar] [CrossRef]
- Chang, L.-L.; Yang, J.; Lai, S.-Q.; Liu, X.-R.; Yang, Z.-W.; Zhao, S.-S. Synthesis, crystal structures and CT-DNA/BSA binding properties of Co(III) and Cu(II) complexes with bipyridine Schiff base ligand. Inorg. Chim. Acta 2022, 532, 120751. [Google Scholar] [CrossRef]
- Obaleye, J.A.; Lawal, M.; Jadeja, R.N.; Gupta, V.K.; Nnabuike, G.G.; Bamigboye, M.O.; Roy, H.; Yusuff, O.K.; Bhagariya, P. Crystal structure, spectroscopic, DFT calculations and antimicrobial study of the Cu(II) complex bearing second-generation quinolone ofloxacin and 2,2′-bipyridine. Inorg. Chim. Acta 2021, 519, 120264. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780521816144. [Google Scholar]
- Figgis, B.N.; Lewis, J. The Magnetic Properties of Transition Metal Complexes. In Progress in Inorganic Chemistry; Cotton, F.A., Ed.; Wiley: Hoboken, NJ, USA, 1964; Volume 6, p. 177192. [Google Scholar] [CrossRef]
- Carlin, R.L.; Burriel, R.; Cornelisse, R.M.; van Duyneveldt, A.J. Magnetic Reinvestigation of [Cu(C5H5NO)2(NO3)2]: Lack of evidence for a Triplet Ground State. Inorg. Chem. 1983, 22, 831–832. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, Q.; Fu, H.; Lin, H.; Luo, C.; Wang, J.; Meng, X.; Peng, H.; Duan, C.-G.; Chu, J. Ferroelectricity and antiferromagnetism in organic–inorganic hybrid (1,4-bis(imidazol-1-ylmethyl)benzene)CuCl4·H2O. Cryst. Eng. Comm. 2020, 22, 587–592. [Google Scholar] [CrossRef]
- Louka, F.R.; Massoud, S.S.; Haq, T.K.; Koikawa, M.; Mikuriya, M.; Omote, M.; Fischer, R.C.; Mautner, F.A. Synthesis, structural characterization and magnetic properties of one-dimensional Cu(II)-azido coordination polymers. Polyhedron 2017, 138, 177–184. [Google Scholar] [CrossRef]
- Kwiatek, D.; Kubicki, M.; Skokowski, P.; Gruszczyńska, J.; Lis, S.; Hnatejko, Z. Five subsequent new pyridine carboxamides and their complexes with d-electron ions. Synthesis, spectroscopic characterization and magnetic properties. J. Mol. Struct. 2019, 1178, 669–681. [Google Scholar] [CrossRef]
- Babu, C.N.; Suresh, P.; Das, P.; Sathyanarayana, A.; Ramadurai, R.; Sampath, N.; Prabusankar, G. Synthesis, crystal structure and spectral properties of copper(II) monomer decorated copper(II) coordination polymer. J. Mol. Struct. 2014, 1062, 141–146. [Google Scholar] [CrossRef]
- Sheldrick, G.M. ShelXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXL-2017/1, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 2017. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, WA, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 6 January 2020).
- Turner, M.J.; Grabowsky, S.; Jayatilaka, D.; Spackman, M.A. Accurate and Efficient Model Energies for Exploring Intermolecular Interactions in Molecular Crystals. J. Phys. Chem. Lett. 2014, 5, 4249–4255. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]








| g‖ | g⊥ | gav | ∆g | HFS b | Line Width c | |
|---|---|---|---|---|---|---|
| [Cu(L1)2(NO3)2] | 2.230 | 2.070 | 2.123 | 0.160 | none | 50, 27, 27 |
| [Cu(L2)2(NO3)2] species I | 2.260 | 2.057 | 2.125 | 0.203 | none | 95, 22, 22 |
| species II | 2.135 | 2.057 | 2.083 | 0.078 | A‖N/Cu = 40 G | 15, 10, 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garci, F.; Chebbi, H.; Rouzbeh, N.; Rochels, L.; Disch, S.; Haseloer, A.; Sebastian, S.S.; Ruschewitz, U.; Anthony, E.T.; Klein, A.; et al. Structure, Optical and Magnetic Properties of Two Isomeric 2-Bromomethylpyridine Cu(II) Complexes [Cu(C6H9NBr)2(NO3)2] with Very Different Binding Motives. Molecules 2023, 28, 731. https://doi.org/10.3390/molecules28020731
Garci F, Chebbi H, Rouzbeh N, Rochels L, Disch S, Haseloer A, Sebastian SS, Ruschewitz U, Anthony ET, Klein A, et al. Structure, Optical and Magnetic Properties of Two Isomeric 2-Bromomethylpyridine Cu(II) Complexes [Cu(C6H9NBr)2(NO3)2] with Very Different Binding Motives. Molecules. 2023; 28(2):731. https://doi.org/10.3390/molecules28020731
Chicago/Turabian StyleGarci, Fatma, Hammouda Chebbi, Nahal Rouzbeh, Leonhard Rochels, Sabrina Disch, Alexander Haseloer, Sean S. Sebastian, Uwe Ruschewitz, Eric Tobechukwu Anthony, Axel Klein, and et al. 2023. "Structure, Optical and Magnetic Properties of Two Isomeric 2-Bromomethylpyridine Cu(II) Complexes [Cu(C6H9NBr)2(NO3)2] with Very Different Binding Motives" Molecules 28, no. 2: 731. https://doi.org/10.3390/molecules28020731
APA StyleGarci, F., Chebbi, H., Rouzbeh, N., Rochels, L., Disch, S., Haseloer, A., Sebastian, S. S., Ruschewitz, U., Anthony, E. T., Klein, A., & Zid, M. F. (2023). Structure, Optical and Magnetic Properties of Two Isomeric 2-Bromomethylpyridine Cu(II) Complexes [Cu(C6H9NBr)2(NO3)2] with Very Different Binding Motives. Molecules, 28(2), 731. https://doi.org/10.3390/molecules28020731