1. Introduction
The medicinal properties of plants can be attributed to the presence of various types of secondary metabolites (or phytoconstituents), such as terpenoids, phenols, flavonoids, and anthraquinones, among others [
1,
2,
3]. These molecules provide plants with antioxidant, anticancer, antimicrobial, antifungal activities, and other beneficial activities [
4,
5,
6,
7,
8], which can be used to treat different diseases and/or conditions [
9]. Plant families that are widely distributed across various environmental conditions or are subjected to biotic and/or abiotic stress are expected to have a diverse range of phytoconstituents. Adverse environmental conditions, such as the presence of pollutants, like heavy metals, can vary the formation of these compounds, and can also alter their content [
10,
11]. This highlights the potential for plants to be a source of novel bioactive compounds that could be used in pharmaceuticals and other applications [
12].
The Asteraceae family comprises a vast number of species, reaching up to 25,037 distributed worldwide, except for the Antarctic continent and Greenland [
13,
14,
15,
16]. Therefore, this family presents high habitat diversity due to the longitudinal and altitudinal gradient, making it a great source of phytoconstituents [
17,
18,
19,
20]. In turn, its
Senecio genus is one of the most diverse among angiosperms [
21] (flowering plants). In Chile, there are around 220 native species of this genus, including 103 endemic species, resulting in a 46% endemism rate [
21]. This genus is subjected to various environmental conditions that make it an excellent reservoir of phytoconstituents. Hence,
Senecio genus possess different medicinal properties, such as anti-inflammatory, antimicrobial, antioxidant, antibacterial, antiviral, antitumor, antiemetic, vasodilator, and analgesic effects. Some of these properties have been useful in treating bronchial asthma, wounds, and other ailments [
6,
22,
23,
24,
25,
26].
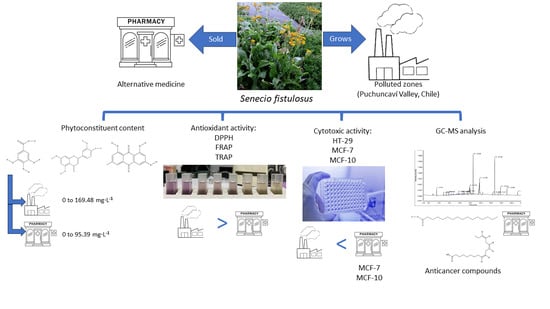
Senecio fistulosus is a perennial herb commonly known as Hualtata or cow’s tongue. It is a perennial herb, robust, with thick rhizomes and large leaves that are arranged in a rosette and can reach a length of 30 to 50 cm. Its stem can grow up in height and ends in a yellow inflorescence. This plant is typically found in the humid areas of central–southern Chile and in the Valparaíso region and is commonly found near wetlands or watercourses, as is the case in the Puchuncaví Valley [
27]. This region is characterized, among other factors, by the presence of a large industrial complex located between the municipalities of Quintero and Puchuncaví. This area has been declared a saturated zone for atmospheric pollutants, including sulfur dioxide (SO
2), respirable particles (PMs), and others. In addition, due to atmospheric depositions, it is common to find elevated concentrations of heavy metals, such as copper (Cu); lead (Pb); zinc (Zn), cadmium (Cd); and metalloids, such as arsenic (As), in the soil and watercourses within this area [
28,
29].
The literature has identified sesquiterpenes with low amounts of pyrrolizidine alkaloids in
S. fistulosus [
30]. Thus, the most frequent chemical groups found in the non-alkaloid fraction are sesquiterpenes of the eremophyllane, furanoeremophyllane, and eremophyllanolide types. Specifically, two of the 9-oxo-furanoeremophyllane type, one of the eremophylanolide type (1
β,10
β-epoxy-6-acetoxy-8
α-hydroxy-eremophyll-7(11)-en-8
β,12-olide), and a maaliol derivative were isolated. In addition, pyrrolizidinic alkaloids were also isolated [
30]. This type of phytoconstituent would have pharmacological properties on the cardiac muscle, highlighting the potential to be antifibrillant. The genus to which it belongs is characterized by possessing a great variety of pyrrolizidinic alkaloids [
30,
31]. For this reason,
S. fistulosus is considered by the Chilean Ministry of Health (Minsal) as a medicinal plant, used for heart disease, bloating, and stomach upset, and is frequently marketed in alternative pharmacies [
27,
31]. Therefore, it is expected that the species obtained from the Puchuncaví valley has a higher content of phytoconstituents, which would lead to higher antioxidant and cytotoxic activity, considering the commercialized species as a positive control.
The aim of this study was to evaluate the effect of industrial pollution in the Puchuncaví valley on the medicinal applications of S. fistulosus, focusing on the phytoconstituent content and its antioxidant and anticarcinogenic activities.
2. Results
2.1. Percentage Yield of Extracts
The percentage of yield obtained from the extracts of the species
S. fistulosus is presented in
Table 1, ranged from 0.35 and 9.24%, where the ethanolic (E) extract stands out as the one with the best yield and the ethyl acetate (A) extract as the one with the lowest yield in both types of samples.
2.2. Photosynthetic Capacity
The photosynthetic capacity of
S. fistulosus was determined only in the Wild species, yielding the results shown in
Table 2, where the effective yield (Yield II) was measured at an irradiance of 1970 µmol photon m
−2 s
−1 at 10:00 am. Also, the maximum yield (
Fv/Fm) obtained after 15 min of shade is observed. In addition, we can find the in situ electron transport rate (ETR in situ) that establishes the productivity of the species under the aforementioned conditions. Finally, the absorptance value is observed, which refers to the amount of light that passes through photosystem two. These data show that the species does not present photoinhibition; therefore, it is possible to point out that the species has a good state of health.
2.3. Phytoconstituents Content
Table 3 shows the content of phenols, flavonoids, and anthraquinones for both Wild and Commercial species obtained in the UV-Vis spectrophotometric assays. According to the statistical analysis, significant differences (
p < 0.05) were observed in these values.
Based on the data presented in
Table 3, the concentration of phytoconstituents analyzed (phenols, flavonoids, and anthraquinones concentrations) varied from 0 to 169.48 mg·L
−1. Medium-polarity extracts (D and A) had the highest content of these metabolites, ranging from 3.60 to 169.48 mg·L
−1, showing differences with the hexane and ethanol extracts, which ranged from 0 to 70.63 mg·L
−1.
It is worth noting that extracts from Wild species had higher contents of phytoconstituents, ranging from 0 to 169.48 mg·L−1, in contrast to the Commercial species, which varied from 0 to 95.38 mg·L−1. The major phytoconstituent found was total phenols among the Wild and Commercial extracts, ranging from 27.53 to 169.48 mg·L−1 and 40.03 to 95.39 mg·L−1, respectively. The second major phytoconstituent found was total flavonoids. These varied between Wild and Commercial extracts from 4.17 to 15.42 mg·L−1 and 1.08 to 13.10 mg·L−1, respectively. Finally, anthraquinones varied from 0 to 31.08 mg·L−1 and from 0 to 9.63 mg·L−1 for Wild and Commercial extracts, respectively. Therefore, it is clear from the results that the Wild species had a higher content of phytoconstituents than the Commercial species and that the medium-polarity solvents had a higher content of phytoconstituents.
2.4. Antioxidant Activity
Table 4 shows the antioxidant activity of different extracts of
S. fistulosus as determined by three antioxidant assays, DPPH, FRAP, and TRAP. According to DPPH assays, a higher IC
50 value indicates lower antioxidant activity. The results show that all DPPH assays measured on the Wild-type and Commercial extracts showed significant differences from the positive controls (Trolox and Gallic acid), presenting values of minimum inhibitory concentration (IC
50) higher than these. However, the medium polarity (D and A) from the Wild-type sample showed values that were closer to those of the positive control (Trolox and Gallic acid). At the same time, extracts D, A, and E from Wild samples had a lower minimum inhibitory concentration (IC
50) (1.89 to 3.29 mg/kg) compared to the stressed samples (4.24 to 9.2 IC
50).
In the case of the FRAP assay, it is observed that extracts H, D, and A of the Wild-type species show higher activity (0.12 to 0.54 TEAC mM) compared to the Commercial species (0.08 to 0.17 TEAC mM), particularly, the medium-polarity (D and A) extracts, which showed values of 0.6 and 0.54 TEAC mM, respectively, for Wild-type and 0.17 and 0.09 TEAC mM, respectively, for Commercial species. In turn, the values obtained in the Wild-type H extract (0.24 TEAC mM) present statistically significant differences compared to the Commercial species (0.08 TEAC mM). The same trend is observed in the TRAP assay, where extracts D, A, and E from the Wild species showed higher antioxidant activity (0.016 TEAC mM in all cases) than Commercial species (0.0032 to 0.0084 TEAC mM), except for extract H. These results suggest that the Wild-type S. fistulosus extracts have higher antioxidant activity than the Commercial samples.
2.5. Correlation between Phytoconstituents Content and Antioxidant Activity
Table 5 presents the level of Spearman’s correlation between the content of phytoconstituents and the antioxidant activity of the extracts being studied. The Spearman correlation evaluates the degree of correspondence between phytoconstituents (measured in terms of phenols, anthraquinones, and flavonoids) and antioxidant activity (measured through various DPPH, FRAP, and TRAP assays). In the case of all variables, the closer it is to 1, the higher the degree of correspondence. However, in the case of DPPH, the correlation values are negative because as the antioxidant activity increases, the IC
50 value decreases, and therefore, it should be close to −1. The results indicate that there is a high correlation between phenol content and FRAP (r > 0.8 and
p < 0.05) and TRAP activity (r > 0.7 and
p < 0.05). Additionally, flavonoids (r > 0.7 and
p < 0.05) and anthraquinones (r > 0.7 and
p < 0.05) show the highest correlation with DPPH stable free radical scavenging activity.
2.6. Analysis of Phytoconstituents by GC-MS
The chemical composition of extracts D and A of Wild and Commercial
S. fistulosus was determined by GC-MS analysis. GC-MS chromatograms are shown in
Figure S1 of the
Supplementary Material. The relative area of the compounds detected in dichloromethane (D) and ethyl acetate (A) extracts is shown in
Table 6.
The extracts consisted mainly of organic compounds of 6 to 20 carbons, including compounds classified as alkaloids, pyrrolizidine alkaloids, diterpenes, aromatics, and saturated and unsaturated fatty acids; these are listed in
Table 6. Extracts D and A of Wild
S. fistulosus showed a similar chemical composition. As for the D and A extracts of Commercial
S. fistulosus, they showed a chemical composition different from each other, and different from the composition of the Wild-type species. The five compounds with the highest relative area of all samples are shown in
Figure 1.
The Wild sample showed between seven and six metabolites (known and unknown) in both extracts, with a percentage area ranging from 42.79% to 1.95%. The major ones (over 10%) are Phytol (1) (42.79 for D and 5.43% for A), Senecionine (2) (40.02% for D and 15.43 for A), Neophytadiene (3) (20.23% for D and 10.93% for A in Wild, 4.31 for D in Commercial), and Indolizine (12.18% for D and 6.33 for A), which dominated the D and A solvent profile (
Figure 1 structures). However, there is a potentially significant compound present in a small proportion (less than 10%): the pyrrolizidine alkaloid Platyphylline. It was identified in the Wild-type species, with a presence of less than 10% (6.44 for D and 1.95 for A). On the other hand, the diacetone alcohol 2-Pentanone. 4-hydroxy-4-methyl- is found in both Wild-type and Commercial species (13.71 for D and 11.60 for A in Wild-type, 2.62 and 2.46 for D and A extracts of the Commercial sample).
When observing the gas chromatogram of the Commercial species, a higher content of compounds (14 and 15 metabolites of known and unknown, respectively) was observed, with a percentage area ranging from 33.10% to 0.59%, of which only Neophytadiene and 2-Pentanone. 4-hydroxy-4-methyl-, the majority, were repeated in the Wild sample, in both cases with a relative area of less than 10% in the Commercial sample.
On the other hand, the metabolites that stand out in the gas chromatogram of the Commercial sample, with a percentage of relative area higher than 10%, in both solvents are (Z,Z,Z) 9,12,15-Octadecatrienoic acid (4) (19.52% for D and 18.36% for A) and n-Hexadecanoic acid (5) (15.72% for D and 12.76% for A) (Structures in
Figure 1).
Table 6 represents the relative amounts of compounds detected in dichloromethane (D) and ethyl acetate (A) extracts of Wild-type and Commercial
S. fistulosus by GC-MS. The compounds shown represent between 70 and 30% of the total compounds, respectively, as they only include compounds with a relative area greater than 10% in any of the extracts and a similarity index (MATCH) greater than 850.
2.7. Cytotoxic Activity
The extracts with the highest phytoconstituent content, and so the highest antioxidant activity, were chosen to evaluate their cytotoxic activity against the HT-29 and MCF-7 tumorigenic, and MCF-10 non-tumorigenic, cellular lines, as presented in
Table 7.
The results presented in
Table 7, show the viability of the HT-29 cancer cell line expressed as a percentage. It can be observed that only the Wild ethyl acetate extract, at a concentration of 100 µg·mL
−1, exhibits significant differences compared to the negative control (C-), which represents the control without any extract. Consequently, the cell viability in the indicated treatment is lower than that of the control. Furthermore, at a concentration of 25 µg·mL
−1, it is observed that significant differences increase compared to the negative control (C-). This suggests that at that concentration, the cancer cells could be using the extract as a source of nutrients, thereby promoting their proliferation.
The viability of the MCF-7 cancer cell line in relation to the two extracts showing the highest antioxidant activity is presented in
Table 7. Notably, the ethyl acetate extract exposed in the Commercial sample with a concentration of 100 µg·mL
−1 stands out, since it demonstrates the lowest percentage of cell viability. This indicates that fewer cells of this cancer type survived this treatment, with only 69.57% of cells remaining viable.
When considering the cell viability of the non-cancerous cell line MCF-10, a remarkable finding is the cytotoxicity exhibited by the ethyl acetate extract of the Commercial S. fistulosus species, specifically, when exposed to a concentration of 100 µg·mL−1. The extract effectively reduced the viability of these cells by almost 50%, showing significant differences compared to both the negative control and the other extracts used in the study. On the other hand, the Wild-type extract proved to be harmless in both healthy and cancer cells.
4. Materials and Methods
4.1. Plants Collection and Extract Preparation
Two specimens of
S. fistulosus, namely, Wild and Commercial, were used in this study for their copper content in the aerial parts non-flowering, including stems and leaves. The Wild sample was located in soil with heavy metals content. The metal content in the topsoil of the sample under study showed the presence of arsenic (15.6 mg·kg
−1), copper (284.7 mg·kg
−1), and lead (16.9 mg·kg
−1). The content of these elements was determined at Silob Chile Laboratory, by SM Method 23rd Edition 3030 K–3125 B; the results of this analysis are presented in the
Supplementary Material. According to previous studies conducted in Puchuncavi valley, the elements in topsoil considered as part of anthropogenic contamination in the soils are As, Cd, Cu, and Pb, with concentrations ranging from 1.5 to 824, 0.01 to 28.4, 8 to 10,000, and 0.74 to 3389 mg·kg
−1, respectively [
28,
55]. The species Wild was collected from the edge of Estuary Puchuncaví, located in the locality of Campiche in the commune of Puchuncaví (coordinates 32.73478° S–71.44666° W), in the region of Valparaíso, Chile, and was taxonomically identified by the curator of the herbarium VALPL of the Universidad de Playa Ancha, MSc. Pamela Ramirez (Voucher VALPL_PLV 2412). On the other hand, the Commercial sample was obtained from an alternative medicine store, where it is sold as dried leaves and stems.
The extract of plant materials (Wild and Commercial) was obtained from 300 g dried leaves and stems under dark and room temperature conditions at the Laboratory of Natural Products and Organic Synthesis (LPNSO), Department of Geography and Natural Sciences, Universidad de Playa Ancha, Valparaíso, Chile. Plant extracts with different organic solvents were obtained using ultrasound equipment (Elma TI-H-5), with two impacts of 1 h each, separated by 24 h. Organic solvents were used in increasing polarity (hexane (H), dichloromethane (D), ethyl acetate (A), and ethanol (E)). The percentage yield has been obtained by Equation (1). Extract concentration was performed in a rotary evaporator (LabTech EV400H) with a vacuum pump (LabTech VP30) at 45 °C for all solvents. These extracts were used to measure phytoconstituent content, antioxidant activity, and cytotoxic activity. For subsequent experiments, the samples were persevered at −4 °C.
The percentage yield (%R) is calculated according to the following equation:
4.2. Photosynthetic Capacity
The photosynthetic capacity of
S. fistulosus was determined only in the Wild species to assess its health status, where the effective quantum yield (Yield II) was measured at an irradiance of 1970 µmol photons m
−2 s
−1 at 10:00 a.m. Furthermore, the maximum yield, the maximum potential of the electron transport chain (
Fv/Fm) obtained after 15 min of shade, was observed [
56].
The in situ electron transport rate (ETR in situ) that determines the in situ productivity in
S. fistulosus under the aforementioned conditions is reported (Equation (2)).
where E is an Irradiance; Yield (II) is photosynthetic capacity in situ; the absorbance value, which refers to the amount of light that passes through photosystem II two, was recorded; and PSII in vascular plants is 0.5.
4.3. Phytoconstituents Determination
The phenol, flavonoid, and anthraquinone components of S. fistulosus different extracts were quantitatively identified by UV–Visible spectrophotometric methods. The content of phytoconstituents and antioxidant activity was measured for extracts with different solvents from the aerial parts of S. fistulosus.
The total phenolic content of the aerial part of the species under study were using the Folin–Ciocalteu colorimetric assay [
57]. Briefly, 0.5 mL of the extract (1 mg·mL
−1 of the dry extract) was added to 2.5 mL of Folin–Ciocalteu reagent (0.2 N), and the solution was mixed thoroughly. After 5 min, 2 mL of a 7.5% sodium carbonate (Na
2CO
3) aqueous solution was added, and the mixture was allowed to stand in the dark for 2 h at room temperature. The absorbance at 700 nm was measured using a UV–visible spectrophotometer (Rayleigh UV-2601). Gallic acid was used as a standard to establish the calibration curve, and the total phenolic content was calculated and expressed as mg of gallic acid equivalents (GAE) per g of dry extract. All samples were worked in triplicate.
The total flavonoid content in the aerial part of
S. fistulosus extract was determined using the aluminum chloride colorimetric method [
57,
58]. In brief, 500 mL of extract solution (1.0 mg·mL
−1 of dry extract) was added to a 2% solution of aluminum chloride in ethanol. After 10 min, the absorbance at 415 nm was measured using a UV–visible spectrophotometer (Rayleigh UV-2601) and a standard solution as against the reagent blank, which consists of 500 mL of the extract with 500 mL of ethanol without AlCl
3. A standard calibration curve was generated using known concentrations of a freshly prepared quercetin solution. Therefore, the total flavonoid concentration in the extract sample was calculated and expressed as mg quercetin equivalents (QEs) per g of dry extract. The assay was carried out in triplicate.
The total anthraquinone content in the aerial part of
S. fistulosus extract was determined using the aluminum chloride colorimetric method [
57], In brief, 500 mL of extract solution (1.0 mg·mL
−1 of dry extract) was added to an equal amount of aluminum chloride solution in 2% ethanol. After 10 min, the absorbance at 485 nm was measured using a UV–visible spectrophotometer (Rayleigh UV-2601) against the reagent blank, which consists of 500 mL of the extract with 500 mL of ethanol without AlCl
3. A standard calibration curve was generated using known concentrations of emodin. Therefore, the total anthraquinone content was calculated and expressed in mg emodin equivalents (EEs) per g dry extract. The estimation of total anthraquinone content was carried out in triplicate.
4.4. Determination of Antioxidant Activity
Different plant extracts obtained were evaluated for their antioxidative activity by three methodologies, 2,2′-diphenyl-1-picrylhydrazyl stable free radical scavenging activity (DPPH∙), ferric reducing power analysis (FRAP), and total reactivity antioxidant potential (TRAP), which are UV–Visible spectrophotometric methods that seek to determine such activity in the extracts under study.
The DPPH∙ free radical scavenging activity was conducted following the method outlined in the current literature [
57,
59]. First, 100 µL of each extract sample (at concentrations of 1.0 mg·mL
−1, 3.0 mg·mL
−1, 5.0 mg·mL
−1, 7.0 mg·mL
−1, and 10.0 mg·mL
−1) was added to 2.9 mL of DPPH solution (concentration of 50 µM) and incubated at room temperature for 15 min. The absorbance at 517 nm was measured spectrophotometrically against a blank sample using the Rayleigh UV-2601 spectrophotometer. The absorbance (Abs.) was measured at zero minutes and after 15 min of incubation with 100 µL of the sample and 2.9 mL of ethanol. All experiments were performed with gallic acid and Trolox
TM used as a positive control. The scavenging activity was determined by calculating the percentage inhibition of the DPPH∙ radical from which the IC
50 value was obtained and reported. All measurements were performed in triplicate.
The ferric reducing power was determined following the methodology outlined in the current literature [
57,
60]. FRAP reagent was prepared by adding 300 mM Buffer solution, 10 mM 2,4,6-tri(2-pyridyl)-s-triacin (TPTZ), and 20 mM ferric chloride, at a ratio of 10:1:1, respectively. Briefly, 100 µL of the sample (which should be at a 1 gr/mL concentration) was added to 3 mL of FRAP reagent and 300 µL of distilled water. The solution was mixed thoroughly and incubated for 30 min. The absorbance at 593 nm was measured spectrophotometrically using the Rayleigh UV-2601 spectrophotometer. Distilled water was blank. A standard calibration curve was generated using known concentrations of Trolox
TM (0–120 mg·mL
−1). The reducing capacity of the extracts was expressed in mM TEAC. All measurements were replicated three times.
The TRAP assay was following the method described in previous studies [
61]. The aim was to evaluate the antioxidant activity of secondary metabolites employing spectrophotometric methods. First, 150 µM of ABTS∙ 2,2’-azino-bis (3-ethylbenzothialin-6-sulfonic acid) (ABTS) and 10 mM of 2,2’-azo-bis (2-amidino propane) (ABAP) were mixed at a 1:1 ratio and incubated with constant stirring at 45 °C for 30 min, followed by cooling. The absorbance at 734 nm was measured spectrophotometrically using the Rayleigh UV-2601 spectrophotometer, every 10 s until 50 s, against a blank solution corresponding to ABTS (the sample contained 1 mL of the mixture and 10 µL of the extract, which were at a 1:100 ratio). A standard calibration curve was generated using known concentrations of Trolox
TM (0–120 mg·mL
−1). The results are expressed in mM Trolox
TM equivalent antioxidant capacity (TEAC μM), using a Trolox
TM standard curve (from 0–120 mg·L
−1). All measurements were repeated three times.
4.5. Cytotoxicity Activity
For the evaluation of the viability of HT-29 and MCF-7 cancer cells and non-tumorigenic line MCF-10, obtained from the Bioassays laboratory of the University of Valparaiso, provided by Dr. Joan Villena, they were treated with the extracts with the highest content of phytoconstituents and antioxidant activity of
S. fistulosus, that is, extracts A and D of both treatments. The sulforhodamine B assay is carried out as expressed in the current literature [
62,
63,
64].
The extracts of S. fistulosus should be kept protected from light and should be worked fresh, and the mother solution should be kept at −20 °C and the working solution at 4 °C. Maintaining these conditions, they should be diluted in dimethyl sulfoxide (DMSO) or ethanol to a concentration of 0.1 M, which will be the stock solution; with this, a concentration of 1 mM will be obtained, which will be the working concentration, from which four different concentrations were made (500 µg·mL−1, 250 µg·mL−1, 125 µg·mL−1, 62.5 µg·mL−1). This should be followed by filtration.
The cell lines used should be washed and trypsinized before reaching 90–100% confluence, a homogeneous 1:1 solution (cell suspension: 0.4% (wt/vol) trypan blue) should be achieved, and the seeding cell density should be at 3 to 5 × 103 cells/well. The cells are incubated at 37 °C, in a 5% CO2 and 95% O2 atmosphere incubator, and treated with the extracts prepared above for 72 h. The cells are then fixed with trichloroacetic acid for 1 h at 4 °C, washed by immersion, stained with 0.1% sulforhodamine B dissolved in acetic acid for 30 min, and washed 3 times, but this time with 1% acetic acid. Finally, the cell density is analyzed in a plate reader at 540 nm, and the viability of the cells under study will be estimated based on the percentage of inhibition. All measurements are in triplicate.
4.6. Analysis of Phytoconstituents by GC-MS Technique
Only the most active extracts (D and A from Wild-type and Commercial species) were analyzed by GC-MS (TRACE 1610, ISQ 7610 mass detector. EEUU). Extracts (1.0 μL) were injected in spitless mode (5 min) on an RTX-5MS column (30 m, 0.25 mm diameter, 5% diphenyl and 95% dimethylpolysiloxane), with helium as the carrier gas at a constant flow rate of 1.5 mL·min−1 and a column pressure of 92.3 KPa. The injector temperature was 230 °C. The thermal profile was as follows: the temperature was held for 3 min at 50 °C and then increased at a rate of 25 °C·min−1 to 250 °C, and then that temperature was held for 15 min.
The mass sweep was operated in electron impact ionization (70 eV), scanning from the m/s range of 30–600 in the full scan mode. The mass spectra were compared with the internal spectra database. Compounds in the chromatograms were identified by comparing their mass spectra with those in the NIST/EPA/NIH mass spectral library [
65]. Chromatographic peaks were considered “unknown” when their similarity index (MATCH) and inverse similarity index (RMATCH) were less than 850, and were discarded [
66]. These parameters refer to the matching ability of the target spectrum to the NIST MS Search 2.4 Library standard spectrum (a value of 1000 indicates a perfect fit).
4.7. Statistical Analysis
Data are reported, using the STATISTICA 7.0 program (StatSoft, Hamburg, Germany), as mean values ± standard deviation (SD). A distribution test was performed to identify the nature of the data obtained from the spectrophotometric tests (phytochemical profile, antioxidant activity, and anticarcinogenic activity). The results of this test for the phenol and flavonoid extracts were found to be parametric, for which they were subjected to an ANOVA test; the rest of the tests were found to be nonparametric, for which the Kruskal–Wallis test was performed for multiple independent variables. In all these cases, the confidence level is 95%. The tests presented a p value < 0.05; therefore, Tukey’s tests were performed. On the other hand, the correlation Spearman Rank Order Correlations between phytoconstituents and antioxidant and anticarcinogenic activity was determined.
5. Conclusions
The main phytoconstituents found in the extracts of the aerial parts of Wild and Commercial S. fistulosus demonstrate a medium polarity. Phenols, followed by flavonoids, are the most abundant phytoconstituents measured, according to UV–Visible spectrophotometric methods, in these extracts. The antioxidant activity of S. fistulosus under abiotic stress exhibits a higher value compared to that reported in other studies involving species from the same genus, and even compared to the Commercial control. The abiotic stress in this study resulted in an increase in the antioxidant activity of S. fistulosus, which is likely the cause of the observed increase in its phytoconstituent content. In the case of the MCF-7 tumorigenic cell line and MCF-10 non-tumorigenic cell line, a cytotoxic effect was observed of S. fistulosus extracts of Commercial species. However, no significant differences were found in the HT-29 cell line. According to GC-MS analysis, molecules such as hexadecanoic acid and (Z,Z,Z)-9,12,15-octadecatrienoic acid could be associated with this cytotoxic activity observed in the Commercial sample.
In conclusion, our study suggests that extracts derived from the aerial parts of S. fistulosus under abiotic stress possess the potential to demonstrate antioxidant activity, over the Commercial control. Therefore, it is important to consider that industrial pollution in the Puchuncaví Valley may have a positive impact on its antioxidant properties, while potentially decreasing its cytotoxic activities, being innocuous for the cancer cell lines and the healthy control used in this study.