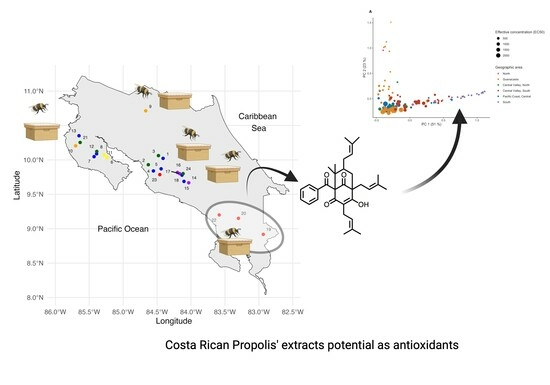
Costa Rican Propolis Chemical Compositions: Nemorosone Found to Be Present in an Exclusive Geographical Zone
Abstract
:1. Introduction
2. Results
2.1. Chemical Spaces of Costa Rican Propolis
2.1.1. Propolis Sampling
2.1.2. NMR Measurements and Data Treatment
2.1.3. Statistical Analysis
2.2. Isolation and Identification of Major Compounds
2.2.1. n-Coniferyl Benzoate
2.2.2. Nemorosone
2.3. Isolation and Characterization of Inactive Components
2.3.1. Agathadiol
| Sample | EC50 DPPH (µg/mL) | ORAC (µmolTE/µmol) | ORAC (µmolTE/mg) |
|---|---|---|---|
| Extracts Apiar 6 (Chemotype I) | 58 ± 19 | Not apply | 1.8 ± 0.2 |
| Extracts Apiar 19 (Chemotype II) | 56 ± 13 | Not apply | 1.7 ± 0.2 |
| Extract Chemotype III | 868 ± 54 | Not apply | <0.1 |
| n-Coniferyl benzoate 1 | 190 ± 10 | 0.60 ± 0.06 | 2.1 ± 0.2 |
| Nemorosone 2 | 300 ± 15 | 0.70 ± 0.2 | 1.4 ± 0.4 |
2.3.2. O-Methyl-n-coniferyl Benzoate
2.4. Antioxidant Activity of Costa Rican Propolis Extracts
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Equipment
4.3. Sampling Strategy
4.4. Preparation of Extracts
4.5. Antioxidant Activity (AOA) Determination
4.6. Sample Preparation for 1H NMR Analysis
4.7. Statistical Analysis
4.8. Isolation of Compounds
4.8.1. Isolation of n-Coniferyl Benzoate (1)
4.8.2. Isolation of Nemorosone (2)
4.8.3. Isolation of DPPH Non-Active Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–16. [Google Scholar] [CrossRef]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complement. Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.-G. Chemical composition of European propolis: Expected and unexpected results. Z. Naturforsch C J. Biosci. 2002, 57, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, É.W.; Negri, G.; Meira, R.M.S.A.; Message, D.; Salatino, A. Plant Origin of Green Propolis: Bee Behavior, Plant Anatomy and Chemistry. Evid. Based Complement. Altern. Med. 2005, 2, 85–92. [Google Scholar] [CrossRef]
- Salatino, A.; Teixeira, É.W.; Negri, G.; Message, D. Origin and Chemical Variation of Brazilian Propolis. Evid. Based Complement. Altern. Med. 2005, 2, 33–38. [Google Scholar] [CrossRef]
- Sawaya, A.; Cunha, I.B.; Marcucci, M.C.; de Oliveira Rodrigues, R.F.; Eberlin, M.N. Brazilian Propolis of Tetragonisca angustula and Apis mellifera. Apidologie 2006, 37, 398–407. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Piccinelli, A.L.; Fernandez, M.C.; Hernández, I.M.; Rosado, A.; Rastrelli, L. Chemical characterization of Cuban propolis by HPLC-PDA, HPLC-MS, and NMR: The brown, red, and yellow Cuban varieties of propolis. J. Agric. Food Chem. 2007, 55, 7502–7509. [Google Scholar] [CrossRef]
- Hernández, I.M.; Fernandez, M.C.; Cuesta-Rubio, O.; Piccinelli, A.L.; Rastrelli, L. Polyprenylated Benzophenone Derivatives from Cuban Propolis. J. Nat. Prod. 2005, 68, 931–934. [Google Scholar] [CrossRef]
- Lotti, C.; Piccinelli, A.L.; Arevalo, C.; Ruiz, I.; De Castro, G.M.M.; De Sá, L.F.R.; Tessis, A.C.; Ferreira-Pereira, A.; Rastrelli, L. Constituents of Hondurian propolis with inhibitory effects on Saccharomyces cerevisiae multidrug resistance protein Pdr5p. J. Agric. Food Chem. 2012, 60, 10540–10545. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Spassov, S.; Tsvetkova, I.; Silva, M.V.; Tsartsarova, M.; Naydenski, C. New bioactive chalcones in propolis from El Salvador. Z. Naturforsch C J. Biosci. 2001, 56, 593–596. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F.; Souza, K.S.; Marcucci, M.C.; Cunha, I.B.S.; Shimizu, M.T. Analysis of the composition of Brazilian propolis extracts by chromatography and evaluation of their in vitro activity against gram-positive bacteria. Braz. J. Microbiol. 2004, 35, 104–109. [Google Scholar] [CrossRef]
- Sawaya, A.; Palma, A.; Caetano, F.; Marcucci, M.; Cunha, I.d.S.; Araujo, C.; Shimizu, M. Comparative study of in vitro methods used to analyse the activity of propolis extracts with different compositions against species of Candida. Lett. Appl. Microbiol. 2002, 35, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs. human colorectal adenocarcinoma cells. Fitoterapia 2015, 106, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Prasain, J.K.; Matsushige, K.; Saiki, I.; Kadota, S. Chemical constituents of Brazilian propolis and their cytotoxic activities. J. Nat. Prod. 1998, 61, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.J.; Santiago, K.B.; Búfalo, M.C.; Herrera, Y.F.; Alday, E.; Velazquez, C.; Hernandez, J.; Sforcin, J.M. Modulatory effects of propolis samples from Latin America (Brazil, Cuba and Mexico) on cytokine production by human monocytes. J. Pharm. Pharmacol. 2015, 67, 1431–1438. [Google Scholar] [CrossRef]
- Hayashi, K.; Komura, S.; Isaji, N.; Ohishi, N.; Yagi, K. Isolation of antioxidative compounds from Brazilian propolis: 3, 4-dihydroxy-5-prenylcinnamic acid, a novel potent antioxidant. Chem. Pharm. Bull. 1999, 47, 1521–1524. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef]
- Nada, A.; Nour, I.; Metwally, A.; Asaad, A.; Shams, S.; Ibrahim, R. An integrated strategy for chemical, biological and palynological standardization of bee propolis. Microchem. J. 2022, 182, 107923. [Google Scholar] [CrossRef]
- Berretta, A.; Nascimiento, A.; Pires, P.; Lima, M.; Marchetti, J. Propolis standardized extract [EEP-AF®] and innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Bobis, O. Plants: Sources of diversity in propolis properties. Plants 2022, 11, 2298. [Google Scholar] [CrossRef]
- Informe de Estado del Ambiente Costa Rica 2017; Ministerio de Ambiente y Energía: San José, Costa Rica, 2018; Capítulo 1: Mensajes clave; pp. 31–33. ISBN 9977-50-148.2.
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; da Rocha Pasin, F.; Tsvetkova, I. Bioactive Constituents of Brazilian Red Propolis. Evid. Based Complement. Altern. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef]
- Watson, D.G.; Peyfoon, E.; Zheng, L.; Lu, D.; Seidel, V.; Johnston, B.; Parkinson, J.A.; Fearnley, J. Application of principal components analysis to 1H-NMR data obtained from propolis samples of different geographical origin. Phytochem. Anal. 2006, 17, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Papotti, G.; Bertelli, D.; Plessi, M.; Rossi, M.C. Use of HR-NMR to classify propolis obtained using different harvesting methods. Int. J. Food Sci. Tech. 2010, 45, 1610–1618. [Google Scholar] [CrossRef]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. 1H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem. Anal. 2012, 23, 260–266. [Google Scholar] [CrossRef]
- Duarte, I.; Barros, A.; Belton, P.S.; Righelato, R.; Spraul, M.; Humpfer, E.; Gil, A.M. High-Resolution Nuclear Magnetic Resonance Spectroscopy and Multivariate Analysis for the Characterization of Beer. J. Agric. Food Chem. 2002, 50, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Kruger, N.J.; Ratcliffe, R.G. Metabolite fingerprinting and profiling in plants using NMR. J. Exp. Bot. 2005, 56, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Popravko, S.A.; Sokolov, I.V.; Torgov, I.V. Derivates of unsaturated aromatic alcohols in propolis and styrax benzoin. Chem. Nat. Compd. 1984, 20, 140–147. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Velez-Castro, H.; Frontana-Uribe, B.A.; Cardenas, J. Nemorosone, the major constituent of floral resins of Clusia rosea. Phytochemistry 2001, 57, 279–283. [Google Scholar] [CrossRef]
- Popova, M.; Chinou, I.; Marekov, I.; Bankova, V. Terpenes with antimicrobial activity from Cretan Propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Frontana-Uribe, B.A.; Ramírez-Apan, T.; Cárdenas, J. Polyisoprenylated benzophenones in cuban propolis; biological activity of nemorosone. Z. Naturforsch C J. Biosci. 2002, 57, 372–378. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy, A critical evaluation of ABTS, DPPH and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Carvalho-Silva, L.B.; Oliveira, M.D.V.; Gontijo, V.S.; Oliveira, W.F.; Derogis, P.B.; Stringheta, P.C.; Nagem, T.J.; Brigagão, M.R.; dos Santos, M.H. Antioxidant, cytotoxic and antimutagenic activities of 7-epi-clusianone obtained from pericarp of Garcinia brasiliensis. Food Res.Int. 2012, 48, 180–186. [Google Scholar] [CrossRef]
- Kim, H.; Kang, M.; Kim, S.; Yim, S.; Lee, I. Bioactive phenolic constituents from culms of Phyllostachys bambusoides. Nat. Prod. Sci. 2011, 17, 267–272. [Google Scholar]
- Hausen, B.M.; Evers, P.; Stüwe, H.T.; König, W.A.; Wollenweber, E. Propolis allergy (IV). Studies with further sensitizers from propolis and constituents common to propolis, poplar buds and balsam of Peru. Contact Derm. 1992, 26, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Myroxylum Balsamum. Available online: http://www.conabio.gob.mx/conocimiento/info_especies/arboles/doctos/30-legum34m.pdf (accessed on 14 June 2021).
- Piccinelli, A.L.; Campone, L.; Piaz, F.D.; Cuesta-Rubio, O.; Rastrelli, L. Fragmentation pathways of polycyclic polyisoprenylated benzophenones and degradation profile of nemorosone by multiple-stage tandem mass spectrometry. J. Am. Soc. Mass. Spectrum. 2009, 20, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- de Castro Ishida, V.F.; Negri, G.; Salatino, A.; Bandeira, M.F. A new type of Brazilian propolis: Prenylated benzophenones in propolis from Amazon and effects against cariogenic bacteria. Food Chem. 2011, 125, 966–972. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Gabbia, D.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Nemorosone inhibits the proliferation and migration of hepatocellular carcinoma cells. Life Sci. 2019, 235, 116817. [Google Scholar] [CrossRef]
- Daniel, W. Bioestadística: Base Para el Análisis de las Ciencias de la Salud; Limusa, S.A. Editorial: Durango, México, 2002; 755p. [Google Scholar]
- Gamez, E.J.; Luyengi, L.; Lee, S.K.; Zhu, L.F.; Zhou, B.N.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, D. Antioxidant flavonoid glycosides from Daphniphyllum calycinum. J. Nat. Prod. 1998, 61, 706–708. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.; Wallet, J.C.; Gaydou, E.M. Antioxidant properties of hydroxy-flavones. Free. Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- Mellors, A.; Tappel, A.L. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J. Biol. Chem. 1966, 241, 4353–4356. [Google Scholar] [CrossRef] [PubMed]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Zamora, L.G.; Beukelman, K.; van den Berg, B.; Arias, M.L.; Umaña, E.; Aguilar, I.; van Ufford, L.Q.; van den Worm, E.; Fallas, N.; Solórzano, R. The antioxidant capacity and immunomodulatory activity of stingless bee honeys proceeding from Costa Rica. Oxid. Antioxid. Med. Sci. 2015, 4, 49–55. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umaña, E.; Solano, G.; Zamora, G.; Tamayo-Castillo, G. Costa Rican Propolis Chemical Compositions: Nemorosone Found to Be Present in an Exclusive Geographical Zone. Molecules 2023, 28, 7081. https://doi.org/10.3390/molecules28207081
Umaña E, Solano G, Zamora G, Tamayo-Castillo G. Costa Rican Propolis Chemical Compositions: Nemorosone Found to Be Present in an Exclusive Geographical Zone. Molecules. 2023; 28(20):7081. https://doi.org/10.3390/molecules28207081
Chicago/Turabian StyleUmaña, Eduardo, Godofredo Solano, Gabriel Zamora, and Giselle Tamayo-Castillo. 2023. "Costa Rican Propolis Chemical Compositions: Nemorosone Found to Be Present in an Exclusive Geographical Zone" Molecules 28, no. 20: 7081. https://doi.org/10.3390/molecules28207081
APA StyleUmaña, E., Solano, G., Zamora, G., & Tamayo-Castillo, G. (2023). Costa Rican Propolis Chemical Compositions: Nemorosone Found to Be Present in an Exclusive Geographical Zone. Molecules, 28(20), 7081. https://doi.org/10.3390/molecules28207081