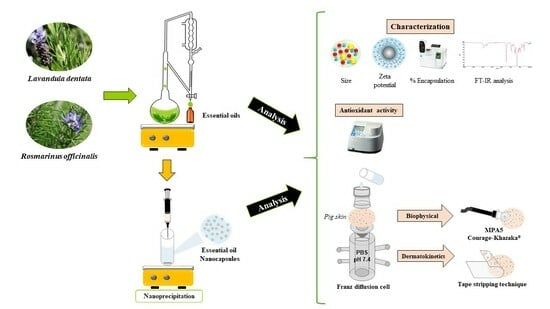
Development of Essential Oil-Loaded Polymeric Nanocapsules as Skin Delivery Systems: Biophysical Parameters and Dermatokinetics Ex Vivo Evaluation
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of EOs
2.2. Preparation and Characterization of the Carrier Systems
2.3. Fourier Transform Infrared (FT-IR) Analysis
2.4. Antioxidant Activity
2.5. Ex Vivo Biophysical Effect on Skin
2.6. Ex Vivo Deposition Studies
3. Discussion
4. Materials and Methods
4.1. Isolation and Physical Characterization of Essential Oils
4.2. Preparation and Characterization of the Carrier Systems
4.3. Fourier Transform Infrared Analysis
4.4. Antioxidant Activity
4.5. Ex Vivo Biophysical Effect on Skin
4.6. Ex Vivo Deposition Studies
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviation
| EOs | essential oils |
| NC-EOs | essential oil-loaded nanocapsules |
| NCs | nanocapsules |
| ROS | reactive oxygen species |
| SC | stratum corneum |
| GC-MS | gas chromatography–mass spectrometry |
| GC-FID | gas chromatography with flame ionization |
| EM-EOs | emulsion with EOs |
| EM-w/o | emulsion without EOs |
| NP-w/o | nanoparticles without EOs |
| FTC | ferric thiocyanate |
| SCWC | stratum corneum water content |
| TEWL | transepidermal water loss |
| FNH | natural hydration factor |
| %E | encapsulation percentage |
| Log P(o/w) | octanol-water partition coefficient |
| PDI | polydispersity index |
| ζ | zeta potential |
References
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of Essential Oils as Skin Permeation Enhancers: Penetration Enhancement Effect and Mechanism of Action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus Officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential Oils Composition in Two Rosmarinus Officinalis L. Varieties and Incidence for Antimicrobial and Antioxidant Activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Shim, C.-H.; Lee, I.-S. Comparative Study of the Chemical Composition and Antioxidant Activity of Six Essential Oils and Their Components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Oh, S.; Zheng, S.; Fang, M.; Kim, M.; Bellere, A.D.; Jeong, J.; Yi, T.H. Anti-Photoaging Effect of Phaseolus Angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials. Molecules 2023, 28, 1407. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Carreño, H.; Stashenko, E.E.; Escobar, P. Essential Oils Distilled from Colombian Aromatic Plants and Their Constituents as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2023, 28, 2872. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; Ribeiro, R.F.; Ourique, A.F.; Rolim, C.M.B.; De Bona Da Silva, C.; Pohlmann, A.R.; Beck, R.C.R.; Guterres, S.S. Nanostructured Systems Containing an Essential Oil: Protection against Volatilization. Quim. Nova 2011, 34, 968–972. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumari, A.; Raina, N.; Zakir, F.; Gupta, M. Prospects of Essential Oil Loaded Nanosystems for Skincare. Phytomed. Plus 2022, 2, 100198. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin Penetration and Distribution of Polymeric Nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Silva-Flores, P.G.; Pérez-López, L.A.; Rivas-Galindo, V.M.; Paniagua-Vega, D.; Galindo-Rodríguez, S.A.; Álvarez-Román, R. Simultaneous GC-FID Quantification of Main Components of Rosmarinus Officinalis L. and Lavandula Dentata Essential Oils in Polymeric Nanocapsules for Antioxidant Application. J. Anal. Methods Chem. 2019, 2019, 2837406. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Lugo-Estrada, L.; Galindo-Rodríguez, S.; Pérez-López, L.; de Torres, N.; Álvarez-Román, R. Headspace–Solid-Phase Microextraction Gas Chromatography Method to Quantify Thymus Vulgaris Essential Oil in Polymeric Nanoparticles. Pharmacogn. Mag. 2019, 15, 473. [Google Scholar] [CrossRef]
- Permanent Commission of the Herbal Pharmacopoeia of the United Mexican States. Farmacopea Herbolaria de Los Estados Unidos Mexicanos, 2nd ed.; Secretaría de Salud: Mexico City, Mexico, 2013. [Google Scholar]
- Atti-Santos, A.C.; Rossato, M.; Pauletti, G.F.; Rota, L.D.; Rech, J.C.; Pansera, M.R.; Agostini, F.; Serafini, L.A.; Moyna, P. Physico-Chemical Evaluation of Rosmarinus officinalis L. Essential Oils. Braz. Arch. Biol. Technol. 2005, 48, 1035–1039. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.M.; et al. Lavandula dentata L.: Phytochemical Analysis, Antioxidant, Antifungal and Insecticidal Activities of Its Essential Oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Krzysko-Lupicka, T.; Pik, A.; Wieczorek, P.P. Chemical Composition of Herbal Macerates and Corresponding Commercial Essential Oils and Their Effect on Bacteria Escherichia Coli. Molecules 2017, 22, 1887. [Google Scholar] [CrossRef] [PubMed]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Rodriguez, S.; Allémann, E.; Fessi, H.; Doelker, E. Physicochemical Parameters Associated with Nanoparticle Formation in the Salting-out, Emulsification-Diffusion, and Nanoprecipitation Methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Enhancement of Topical Delivery from Biodegradable Nanoparticles. Pharm. Res. 2004, 21, 1818–1825. [Google Scholar] [CrossRef]
- Pina-Barrera, A.M.; Alvarez-Roman, R.; Baez-Gonzalez, J.G.; Amaya-Guerra, C.A.; Rivas-Morales, C.; Gallardo-Rivera, C.T.; Galindo-Rodriguez, S.A. Application of a Multisystem Coating Based on Polymeric Nanocapsules Containing Essential Oil of Thymus Vulgaris L. to Increase the Shelf Life of Table Grapes (Vitis Vinifera L.). IEEE Trans Nanobiosci. 2019, 18, 549–557. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible Films from Essential-Oil-Loaded Nanoemulsions: Physicochemical Characterization and Antimicrobial Properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery. Methods in Molecular Biology; McNeil, S., Ed.; Humana Press: New York, NY, USA, 2011; Volume 697. [Google Scholar] [CrossRef]
- Linares, V.; Yarce, C.J.; Echeverri, J.D.; Galeano, E.; Salamanca, C.H. Relationship between Degree of Polymeric Ionisation and Hydrolytic Degradation of Eudragit® E Polymers under Extreme Acid Conditions. Polymers 2019, 11, 1010. [Google Scholar] [CrossRef]
- Etman, M.A.; Gamal, M.; Nada, A.H.; Shams-Eldeen, M.A. Formulation of Desloratadine Oral Disintegrating Tablets. J. Appl. Pharm. Sci. 2014, 4. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- de Moraes, Â.A.B.; Ferreira, O.O.; da Costa, L.S.; Almeida, L.Q.; Varela, E.L.P.; Cascaes, M.M.; de Jesus Pereira Franco, C.; Percário, S.; do Nascimento, L.D.; de Oliveira, M.S.; et al. Phytochemical Profile, Preliminary Toxicity, and Antioxidant Capacity of the Essential Oils of Myrciaria Floribunda (H. West Ex Willd.) O. Berg. and Myrcia Sylvatica (G. Mey) DC. (Myrtaceae). Antioxidants 2022, 11, 2076. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant Activity of Clove Oil—A Powerful Antioxidant Source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in Human Skin: Organ-Specific Physiology and Considerations for Its Use in Dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Rocha Caldas, G.F.; Oliveira, A.R.D.S.; Araújo, A.V.; Lafayette, S.S.L.; Albuquerque, G.S.; Silva-Neto, J.D.C.; Costa-Silva, J.H.; Ferreira, F.; Da Costa, J.G.M.; Wanderley, A.G. Gastroprotective Mechanisms of the Monoterpene 1,8-Cineole (Eucalyptol). PLoS ONE 2015, 10, e0134558. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, I.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity-Structure Relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Hosni, K.; Jemli, M.; Dziri, S.; M’rabet, Y.; Ennigrou, A.; Sghaier, A.; Casabianca, H.; Vulliet, E.; Ben Brahim, N.; Sebei, H. Changes in Phytochemical, Antimicrobial and Free Radical Scavenging Activities of the Peruvian Pepper Tree (Schinus Molle L.) as Influenced by Fruit Maturation. Ind. Crop. Prod. 2011, 34, 622–1628. [Google Scholar] [CrossRef]
- Cauchetier, E.; Deniau, M.; Fessi, H.; Astier, A.; Paul, M. Atovaquone-Loaded Nanocapsules: Influence of the Nature of the Polymer on Their in Vitro Characteristics. Int. J. Pharm. 2003, 250, 273–281. [Google Scholar] [CrossRef]
- Contri, R.V.; Fiel, L.A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Transport of Substances and Nanoparticles across the Skin and in Vitro Models to Evaluate Skin Permeation and/or Penetration. In Nanocosmetics and Nanomedicines; Springer: New York, NY, USA, 2011; pp. 3–35. [Google Scholar]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In Vitro Skin Models as a Tool in Optimization of Drug Formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef]
- Logger, J.; Olydam, J.; Woliner-van der Weg, W.; van Erp, P. Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study. Cosmetics 2019, 6, 20. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Dermatopharmacokinetic Prediction of Topical Drug Bioavailability In Vivo. J. Investig. Dermatol. 2007, 127, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Janicki, S.; Sznitowska, M. In Vitro Studies on Penetration of Terpenes from Matrix-Type Transdermal Systems through Human Skin. Int. J. Pharm. 2001, 14, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Roberts, M.S. Dermatologic, Cosmeceutic, and Cosmetic Development: Therapeutic and Novel Approaches, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Robinson, M.; Visscher, M.; Laruffa, A.; Wickett, R. Abstracts: Natural Moisturizing Factors in the Stratum Corneum I. Effects of Lipid Extraction and Soaking. Int. J. Cosmet. Sci. 2010, 32, 394. [Google Scholar] [CrossRef]
- Jonsdottir, F.; Snorradottir, B.S.; Gunnarsson, S.; Georgsdottir, E.; Sigurdsson, S. Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics 2022, 14, 1880. [Google Scholar] [CrossRef]
- Permanent Commission of Pharmacopoeia of the United Mexican States. Farmacopea de Los Estados Unidos Mexicanos, 10th ed.; Secretaría de Salud: Mexico City, Mexico, 2011. [Google Scholar]
- Lugo Estrada, L. Obtención y Caracterización de Nanopartículas Poliméricas Para La Encapsulación de Aceites Esenciales Por La Técnica de Nanoprecipitación; UANL: Monterrey, Mexico, 2012. [Google Scholar]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin Physiology in Men and Women: In Vivo Evaluation of 300 People Including TEWL, SC Hydration, Sebum Content and Skin Surface PH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef]








| EO | Refractive Index 1 | Relative Density (g/mL) 1 | Optical Rotation (°) 2 |
|---|---|---|---|
| R. officinalis | 1.469 ± 0.000 | 0.894 ± 0.002 | +11.80 ± 0.01 |
| L. dentata | 1.470 ± 0.000 | 0.900 ± 0.002 | −1.67 ± 0.01 |
| NC-EO | Mean Size (nm) | PDI | Zeta Potential (mV) | pH | %E | |
|---|---|---|---|---|---|---|
| NC-R. officinalis | 227.73 ± 2.96 | 0.20 ± 0.03 | 54.47 ± 0.45 | 6.28 ± 0.06 | 1,8-cineole | 2.95 ± 0.14 |
| Camphor | 2.41 ± 0.13 | |||||
| NC-L. dentata | 230.99 ± 8.85 | 0.22 ± 0.03 | 50.40 ± 0.75 | 6.66 ± 0.02 | β-pinene | 1.56 ± 0.13 |
| 1,8-cineole | 2.89 ± 0.12 | |||||
| 15 µg/mL | 30 µg/mL | 45 µg/mL | |
|---|---|---|---|
| α-tocopherol | 60.83 ± 0.86 | 64.05 ± 0.67 | 68.01 ± 0.59 |
| 1,8-cineole | 60.60 ± 0.81 | 63.99 ± 0.57 | 65.36 ± 1.05 |
| Camphor | 61.79 ± 0.86 | 64.32 ± 0.95 | 70.83 ± 0.90 |
| EO-R. officinalis | 59.85 ± 1.18 | 63.21 ± 1.01 | 65.21 ± 0.66 |
| EO-L. dentata | 60.21 ± 0.52 | 63.33 ± 0.95 | 66.79 ± 0.67 |
| NC-R. officinalis | 56.28 ± 0.72 | 59.97 ± 0.58 | 61.70 ± 0.67 |
| NC-L. dentata | 57.59 ± 0.82 | 60.57 ± 0.63 | 61.93 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Flores, P.G.; Galindo-Rodríguez, S.A.; Pérez-López, L.A.; Álvarez-Román, R. Development of Essential Oil-Loaded Polymeric Nanocapsules as Skin Delivery Systems: Biophysical Parameters and Dermatokinetics Ex Vivo Evaluation. Molecules 2023, 28, 7142. https://doi.org/10.3390/molecules28207142
Silva-Flores PG, Galindo-Rodríguez SA, Pérez-López LA, Álvarez-Román R. Development of Essential Oil-Loaded Polymeric Nanocapsules as Skin Delivery Systems: Biophysical Parameters and Dermatokinetics Ex Vivo Evaluation. Molecules. 2023; 28(20):7142. https://doi.org/10.3390/molecules28207142
Chicago/Turabian StyleSilva-Flores, Perla Giovanna, Sergio Arturo Galindo-Rodríguez, Luis Alejandro Pérez-López, and Rocío Álvarez-Román. 2023. "Development of Essential Oil-Loaded Polymeric Nanocapsules as Skin Delivery Systems: Biophysical Parameters and Dermatokinetics Ex Vivo Evaluation" Molecules 28, no. 20: 7142. https://doi.org/10.3390/molecules28207142
APA StyleSilva-Flores, P. G., Galindo-Rodríguez, S. A., Pérez-López, L. A., & Álvarez-Román, R. (2023). Development of Essential Oil-Loaded Polymeric Nanocapsules as Skin Delivery Systems: Biophysical Parameters and Dermatokinetics Ex Vivo Evaluation. Molecules, 28(20), 7142. https://doi.org/10.3390/molecules28207142