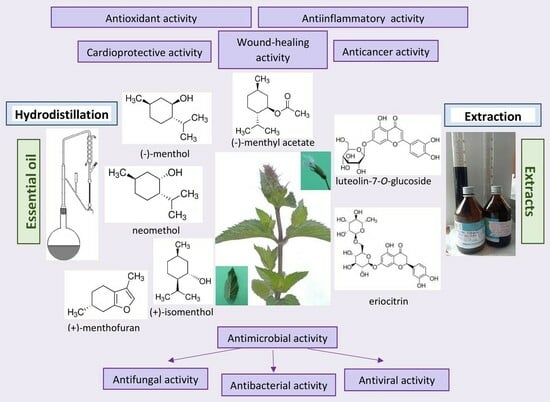
Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products
Abstract
:1. Introduction
2. Historical, Botanical, and Taxonomic Characteristics of Mentha piperita
3. Chemical Composition of Essential Oil
4. Chemical Composition of Extracts of Mentha piperita
5. Antioxidant Activity
6. Anti-Inflammatory Activity
7. Analgesic Activity
8. Antimicrobial Activity
8.1. Antiviral Activity
8.2. Antibacterial and Antifungal Activities
9. Anticancer Activity
10. Cardiovascular Diseases
11. Other Activities (Gastrointestinal Effects, Protective Activity, Larvicidal, and Repellent Activities) and Other Applications
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nilo, M.C.S.; Riachi, L.G.; Simas, D.L.R.; Coleho, G.C.; da Silva, A.J.R.; Costa, D.C.M.; Alviano, D.S.; Alviano, C.S.; De Maria, C.A.B. Chemical composition and antioxidant and antifungal properties of Mentha × piperita L. (peppermint) and Mentha arvensis L. (cornmint) samples. Food Res. 2017, 1, 147–156. [Google Scholar] [CrossRef]
- Hudz, N.; Ivanova, R.; Brindza, J.; Grygorieva, O.; Schubertová, Z.; Ivanišová, E. Approaches to the determination of antioxidant activity of extracts from bee bread and Safflower leaves and flowers. Potravin. Slovak J. Food Sci. 2017, 11, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Yezerska, O.; Grygorieva, O.; Brindza, J.; Felsöciová, S.; Kačániová, M.; Wieczorek, P. Analytical procedure elaboration of total flavonoid content determination and antimicrobial activity of bee bread extracts. Acta Pol. Pharm. Drug Res. 2019, 76, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Makowicz, E.; Shanaida, M.; Białoń, M.; Jasicka-Misiak, I.; Yezerska, O.; Svydenko, L.; Wieczorek, P.P. Phytochemical evaluation of tinctures and essential oil obtained from Satureja montana herb. Molecules 2020, 25, 4763. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical variability and pharmacological potential of propolis as a source for the development of new pharmaceutical products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Hudz, N.; Białoń, M.; Kryvtsowa, M.; Svydenko, L.; Filipska, A.; Wieczorek, P.P. Chromatographic profiles and antimicrobial activity of the essential oils obtained from some species and cultivars of the Mentheae tribe (Lamiaceae). Saudi J. Biol. Sci. 2021, 28, 6145–6152. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- El-Lateef Gharib, F.A.; da Silva, J.A.T. Composition, total phenolic content and antioxidant activity of the essential oil of four Lamiaceae herbs. Med. Aromat. Plant Sci. Biotechnol. 2013, 7, 19–27. [Google Scholar]
- Gonçalves, R.S.; Battistin, A.; Pauletti, G.; Rota, L.; Serafini, L.A. Antioxidant properties of essential oils from Mentha species evidenced by electrochemical methods. Rev. Bras. Plantas Med. 2009, 11, 372–382. [Google Scholar] [CrossRef]
- Çolak, E.B.; Çobanoğlu, Ö.; Tepecik, M.; Öztürk, B.; Anaç, D. Yield, essential nutrients and essential oils of peppermint (Mentha × piperita L.) grown under organic farming conditions. J. Agric. 2015, 29, 29–36. [Google Scholar]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical composition of the essential oil of the new cultivars of Lavandula angustifolia mill. bred in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Geuenich, S.; Goffinet, C.; Venzke, S.; Nolkemper, S.; Baumann, I.; Plinkert, P.; Reichling, J.; Keppler, O.T. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-hiv-1 activity by increasing the virion density. Retrovirology 2008, 5, 27. [Google Scholar] [CrossRef]
- Yarnell, E. Herbs for viral respiratory infections. Altern. Complement. Ther. 2018, 24, 35–43. [Google Scholar] [CrossRef]
- Zaidi, S.; Dahiya, P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. Int. Food Res. J. 2015, 22, 2440–2445. [Google Scholar]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant Activity of Rosmarinic Acid Extracted and Purified from Mentha piperita. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC8934100 (accessed on 7 August 2023).
- Srivastava, S.N.; Dixit, D. In vitro antioxidant and antimicrobial activities of herbal combinations of Rosemarinus officinalis (rosemary) and Mentha piperita (peppermint). Int. J. Biol. Pharm. Allied Sci. 2021, 10, 2974–2986. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE. 2014, 9, e114767. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Uribe, E.; Marín, D.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, A. Assessment of vacuum-dried peppermint (Mentha piperita L.) as a source of natural antioxidants. Food Chem. 2016, 190, 559–565. [Google Scholar] [CrossRef]
- Kiełtyka-Dadasiewicz, A. Morphological and genetic diversity among peppermint (Mentha × piperita L.) cultivars. Acta Sci. Pol. Hortorum Cultus 2017, 16, 151–161. [Google Scholar] [CrossRef]
- Abbas, S.; Sultana, S.; Chishti, A.W.; Akram, M.; Ali Shah, S.M.A.; Sareen, A.; Siddique, S.; Aftab, A. Mentha piperita: Medicinal uses and pharmacological properties. Int. J. Sch. Res. Biol. Pharm. 2022, 1, 041–045. [Google Scholar] [CrossRef]
- Shabir Ahmad, R.; Imran, A.; Sajid Arshad, M.; Bilal Hussain, M.; Waheed, M.; Safdar, S.; Yasmin, Z. Introductory chapter: Mentha piperita (a valuable herb): Brief overview. Herbs Spices 2020. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Samber, N.; Manzoor, N. Antimicrobial activity of Mentha piperita essential oil in combination with silver ions. Synergy 2014, 1, 92–98. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Machado, F.M.; Oshiiwa, M.; Abreu, M.; Guiger, E.L.; Tomazela, P.; Goulart, R.A. Investigation of the effects of peppermint (Mentha piperita) on the biochemical and anthropometric profile of university students. Ciência E Tecnol. Aliment. 2011, 31, 584–588. [Google Scholar] [CrossRef]
- Sústriková, A.; Šalamon, I. Essential oil of peppermint (Mentha × piperita L.) from fields in eastern Slovakia. Hortic. Sci. 2004, 31, 31–36. [Google Scholar] [CrossRef]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid screening of Mentha spicata essential oil and L-menthol in Mentha piperita essential oil by ATR-ftir spectroscopy coupled with multivariate analyses. Foods 2021, 10, 202. [Google Scholar] [CrossRef]
- Patrignani, F.; Prasad, S.; Novakovic, M.; Marin, P.D.; Bukvicki, D. Lamiaceae in the treatment of cardiovascular diseases. Front. Biosci. 2021, 26, 612–643. [Google Scholar] [CrossRef]
- Rubio, C.; Lucas, J.R.D.; Gutiérrez, A.J.; Glez-Weller, D.; Pérez Marrero, B.; Caballero, J.M.; Revert, C.; Hardisson, A. Evaluation of metal concentrations in Mentha herbal teas (Mentha piperita, Mentha pulegium and Mentha species) by inductively coupled plasma spectrometry. J. Pharm. Biomed. Anal. 2012, 71, 11–17. [Google Scholar] [CrossRef]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef]
- Soković, M.; Vukojević, J.; Marin, P.; Brkić, D.; Vajs, V.; Van Griensven, L. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- ESCOP European Scientific Cooperative on Phytotheraphy. E/S/C/O/P Monographs Menthae Piperitea Folium; ESCOP Monographs, 2019; Available online: https://escop.com/wp-content/uploads/edd/2019/03/Menthae-piperitae-folium-ESCOP-2019.pdf (accessed on 25 September 2023).
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Mahendran, G.; Rahman, L. Ethnomedicinal, phytochemical and pharmacological updates on peppermint (Mentha piperita)—A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and health attributes to treat various ailments including cardiovascular diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef] [PubMed]
- American Botanical Council. Expanded Commission E Monographs.Peppermint Oil [WWW Document]. 2023. Available online: https://www.herbalgram.org/resources/expanded-commission-e-monographs/peppermint-oil/ (accessed on 25 September 2023).
- Kalemba, D.; Synowiec, A. Agrobiological interactions of essential oils of two menthol mints: Mentha piperita and Mentha arvensis. Molecules 2019, 25, 59. [Google Scholar] [CrossRef]
- Rachwalik, R.; Kurowski, G.; Vogt, E.; Vogt, O. Technologies of Essential Oils; Wydawnictwo PK: Kraków, Poland, 2020. [Google Scholar]
- Silva, H. A descriptive overview of the medical uses given to Mentha aromatic herbs throughout history. Biology 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the Project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Ferreira, P.; Cardoso, T.; Ferreira, F.; Fernandes-Ferreira, M.; Piper, P.; Sousa, M.J. Mentha piperita essential oil induces apoptosis in yeast associated with both cytosolic and mitochondrial Ros-mediated damage. FEMS Yeast Res. 2014, 14, 1006–1014. [Google Scholar] [CrossRef]
- Khorasaninejad, S.; Mousavi, A.; Soltanloo, H.; Hemmati, K.; Khalighi, A. The effect of salinity stress on growth parameters, essential oil yield and constituent of peppermint (Mentha piperita L.). World Appl. Sci. J. 2010, 11, 1403–1407. [Google Scholar]
- Lawrence, B.M. Mint: The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Abdi, G.; Shokrpour, M.; Karami, L.; Salami, S.A. Prolonged Water Deficit Stress and Methyl Jasmonate-Mediated Changes in Metabolite Profile, Flavonoid Concentrations and Antioxidant Activity in Peppermint (Mentha × piperita L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 70–80. [Google Scholar] [CrossRef]
- El Hassani, F.Z. Characterization, activities, and ethnobotanical uses of Mentha species in Morocco. Heliyon 2020, 6, e05480. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of genus Mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.O. Mentha: Economic uses. In Mint: The Genus Mentha; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 519–522. [Google Scholar] [CrossRef]
- Dostál, J. Nová květena ČSSR [New flowers of CSR]; Academia: Praha, Czech Republic, 1989. [Google Scholar]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and antimicrobial characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential oils and in vitro potential cytotoxic effect in human colorectal carcinoma cells. Molecules 2022, 27, 6106. [Google Scholar] [CrossRef]
- Arrahmouni, R.; Ouazzani, C.; Er-Ramly, A.; Moustaghfir, A.; Dami, A.; Ballouch, L. Chemical composition of Moroccan commercial essential oils of mint: Mentha spicata, Mentha piperita, and Mentha pulegium. Trop. J. Nat. Prod. Res. 2023, 7, 2708–2712. [Google Scholar] [CrossRef]
- Góra, J.; Lis, A. Najcenniejsze Olejki Eteryczne Część I, 4th ed.; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2019. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (Ph. Eur.), 11th ed.; Strasbourg: Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Scavroni, J.; Boaro, C.S.; Marques, M.O.; Ferreira, L.C. Yield and composition of the essential oil of Mentha piperita L. (Lamiaceae) grown with biosolid. Braz. J. Plant Physiol. 2005, 17, 345–352. [Google Scholar] [CrossRef]
- Tepecik, M.; Esetlili, B.Ç.; Oztürk, B.; Anaç, D. Effect of different fertilizers on peppermint—Essential and non-essential nutrients, essential oils and yield. Ital. J. Agron. 2022, 17, 1. [Google Scholar] [CrossRef]
- Łyczko, J.; Piotrowski, K.; Kolasa, K.; Galek, R.; Szumny, A. Mentha piperita L. Micropropagation and the Potential Influence of Plant Growth Regulators on Volatile Organic Compound Composition. Molecules 2020, 25, 2652. [Google Scholar] [CrossRef]
- Al-Amri, S.M. Response of growth, essential oil composition, endogenous hormones and microbial activity of Mentha piperita to some organic and biofertilizers agents. Saudi J. Biol. Sci. 2021, 28, 5435–5441. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Menthofuran regulates essential oil biosynthesis in peppermint by controlling a downstream monoterpene reductase. Proc. Natl. Acad. Sci. USA 2003, 100, 14481. [Google Scholar] [CrossRef]
- Verma, R.S.; Rahman, L.U.; Verma, R.K.; Chauhan, A.; Yadav, A.K.; Singh, A. Essential Oil Composition of Menthol Mint (Mentha arvensis and Peppermint (Mentha piperita) Cultivars at Different Stages of Plant Growth from Kumaon Region of Western Himalaya. J. Med. Aromat. Plants 2010, 1, 13–18. [Google Scholar]
- Kehili, S.; Boukhatem, M.N.; Belkadi, A.; Ferhat, M.A. Peppermint (Mentha piperita L.) essential oil as a potent anti-inflammatory, wound healing and anti-nociceptive drug. Eur. J. Biol. Res. 2020, 10, 132–149. [Google Scholar]
- Kizil, S.; Haşimi, N.; Tolan, V.; Kilinç, E.; Yüksel, U. Mineral content, essential oil components and biological activity of two mentha species (M. piperita L., M. spicata L.). Turk. J. Field Crops 2010, 15, 148–153. [Google Scholar]
- Hamad Al-Mijalli, S.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid. Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha × piperita. Nat. Prod. Commun. 2009, 4, 1934578X0900400819. [Google Scholar] [CrossRef]
- Chalchat, J.-C.; Garry, R.-P.; Michet, A. Variation of the chemical composition of essential oil of Mentha piperita L. during the growing time. J. Essent. Oil Res. 1997, 9, 463–465. [Google Scholar] [CrossRef]
- Watson, V.K.; St. John, J.L. Peppermint oil, relation of maturity and curing of peppermint hay to yield and composition of oil. J. Agric. Food Chem. 1955, 3, 1033–1038. [Google Scholar] [CrossRef]
- Piccaglia, R.; Dellacecca, V.; Marotti, M.; Giovanelli, E. Agronomic factors affecting the yields and the essential oil composition of peppermint (Mentha × piperita L.). Acta Hortic. 1993, 344, 29–40. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in Essential Oils. Peppermint oil. Perfum. Flavourist 2013, 38, 47–51. [Google Scholar]
- Beigi, M.; Torki-Harchegani, M.; Ghasemi Pirbalouti, A. Quantity and chemical composition of essential oil of peppermint (Mentha × piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef]
- Hawrył, M.A.; Skalicka-Woźniak, K.; Świeboda, R.; Niemiec, M.; Stępak, K.; Waksmundzka-Hajnos, M.; Hawrył, A.; Szymczak, G. GC-MS Fingerprints of mint essential oils. Open Chem. 2015, 13, 000010151520150148. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Qiu, J.-Z.; Wang, J.-F.; Luo, M.-J.; Li, H.-E.; Leng, B.-F.; Ren, W.-Z.; Deng, X.-M. Peppermint oil decreases the production of virulence-associated exoproteins by Staphylococcus aureus. Molecules 2011, 16, 1642–1654. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Wu, K.; Yu, G.; Liu, C.; Su, C.; Yi, F. Study on the Effect of Mentha × piperita L. Essential Oil on Electroencephalography upon Stimulation with Different Visual Effects. Molecules 2022, 27, 4059. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Eccles, R. Menthol and related cooling compounds. J. Pharm. Pharmacol. 1994, 46, 618–630. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Q.; Shang, Z.; Liu, J.; Zhou, S.; Dang, J.; Liu, L.; Lange, I.; Srividya, N.; Lange, B.M.; et al. Functional characterization and structural insights into stereoselectivity of pulegone reductase in menthol biosynthesis. Front. Plant Sci. 2021, 12, 780970. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Public Statement on the Use of Herbal Medicinal Products Containing Pulegone and Mentophurane; EMA: London, UK, 2016; Volume 44, pp. 1–24. [Google Scholar]
- Wei, H.; Kong, S.; Jayaraman, V.; Selvaraj, D.; Soundararajan, P.; Manivannan, A. Mentha arvensis and Mentha × piperita-Vital Herbs with Myriads of Pharmaceutical Benefits. Horticulturae 2023, 9, 224. [Google Scholar] [CrossRef]
- Khalil, A.F.; Elkatry, H.O.; El Mehairy, H.F. Protective effect of peppermint and parsley leaves oils against hepatotoxicity on experimental rats. Ann. Agric. Sci. 2015, 60, 353–359. [Google Scholar] [CrossRef]
- Tsai, M.; Wu, C.; Lin, T.; Lin, W.; Huang, Y.; Yang, C. Chemical composition and biological properties of essential oils of two mint species. Trop. J. Pharm. Res. 2013, 12, 577–582. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- ISO. ISO 856 Oil of Peppermint (Mentha × piperita L.); International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- WHO. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- United States Pharmacopeial Convention. The United States Pharmacopeia 42. National Formulary 37; United States Pharmacopeial Convention: North Bethesda, MD, USA, 2019. [Google Scholar]
- Arruda, M.O.; Mendes, S.J.; Teixeira, S.A.; de Mesquita, L.S.; de Sousa Ribeiro, M.N.; de Galvão, S.; Muscará, M.N.; Fernandes, E.S.; Monteiro-Neto, V. The hydroalcoholic extract obtained from Mentha piperita L. leaves attenuates oxidative stress and improves survival in lipopolysaccharide-treated macrophages. J. Immunol. Res. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Koşar, M.; Başer, K.H.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha × piperita L. (peppermint) extracts. Nat. Prod. Commun. 2009, 4, 1934578X0900400419. [Google Scholar] [CrossRef]
- Aliki, T.; Archountoula, C.; Evaggelos, Z.; Panagiotis, H.; Dimitra, H. Antioxidant activity of mint (Mentha piperita L.) of Greek flora and identification of its bioactive compounds. Org. Med. Chem. 2021, 11. [Google Scholar] [CrossRef]
- Mallick, B.; Sinha, S.; Roy, D. Evaluation of antioxidative potential of field grown and tissue culture derived Mentha piperita L. plants. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 382–391. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. [Google Scholar] [PubMed]
- Athanasiadis, V.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Β-cyclodextrin-aided aqueous extraction of antioxidant polyphenols from peppermint (Mentha × piperita L.). Oxygen 2022, 2, 424–436. [Google Scholar] [CrossRef]
- Jamshed, A.; Jabeen, Q. Pharmacological evaluation of Mentha piperita against urolithiasis: An in vitro and in vivo study. Dose-Response 2022, 20, 155932582110730. [Google Scholar] [CrossRef]
- Alexa, E.; Danciu, C.; Radulov, I.; Obistioiu, D.; Sumalan, R.M.; Morar, A.; Dehelean, C.A. Phytochemical screening and biological activity of Mentha × piperita L. and Lavandula angustifolia mill. extracts. Anal. Cell. Pathol. 2018, 2018, 2678924. [Google Scholar] [CrossRef]
- Zaia, M.G.; di Cagnazzo, T.; Feitosa, K.A.; Soares, E.G.; Faccioli, L.H.; Allegretti, S.M.; Afonso, A.; de Anibal, F. Anti-inflammatory properties of menthol and menthone in Schistosoma mansoni infection. Front. Pharmacol. 2016, 7, 170. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ben Hsouna, A.; Athmouni, K.; van Pelt, J.; Makni Ayadi, F.; Rebai, T.; Elfeki, A. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- İşcan, G.; Kïrïmer, N.; Kürkcüoǧlu, M.; Başer, K.H.C.; Demïrcï, F. Antimicrobial Screening of Mentha piperita Essential Oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef]
- Keshavarz Mirzamohammadi, H.; Modarres-Sanavy, S.A.; Sefidkon, F.; Mokhtassi-Bidgoli, A.; Mirjalili, M.H. Irrigation and fertilizer treatments affecting rosmarinic acid accumulation, total phenolic content, antioxidant potential and correlation between them in Peppermint (Mentha piperita L.). Irrig. Sci. 2021, 39, 671–683. [Google Scholar] [CrossRef]
- Hudz, N.; Turkina, V.; Yezerska, O.; Kobylinska, L.; Filipska, A.; Karosiene, J.; Galinytė, D.; Balciunaite–Murziene, G. Evaluation of the spectral characteristics, purity and antioxidant activity of C-phycocyanin from the cyanobacteria collected in Kaunas Lagoon (Lithuania). Ukr. Biochem. J. 2022, 94, 47–58. [Google Scholar] [CrossRef]
- Elmastaş, M.; Telci, İ.; Akşit, H.; Erenler, R. Comparison of total phenolic contents and antioxidant capacities in mint genotypes used as spices/baharat olarak Kullanılan Nane genotiplerinin toplam Fenolik içerikleri ve Antioksidan Kapasitelerinin Karşılaştırılması. Turk. J. Biochem. 2015, 40, 456–462. [Google Scholar] [CrossRef]
- Nickavar, B.; Alinaghi, A.; Kamalinejad, M. Evaluation of the Antioxidant Properties of Five Mentha Species. Iran. J. Pharm. Res. 2008, 7, 203–209. [Google Scholar]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.; Rehman, N. Mentha: A genus rich in vital nutra-pharmaceuticals—A Review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef]
- Kehili, S.; Boukhatem, M.A.; Belkadi, A.; Boulaghmen, F.; Ferhat, M.A.; Setzer, W.N. Spearmint (Mentha spicata L.) essential oil from Tipaza (Algeria): In vivo anti-inflammatory and analgesic activities in experimental animal models. Acta Pharm. Hung. 2020, 90, 15–26. [Google Scholar] [CrossRef]
- Cosentino, M.; Bombelli, R.; Conti, A.; Colombo, M.L.; Azzetti, A.; Bergamaschi, A.; Marino, F.; Lecchini, S. Antioxidant properties and in vitro immunomodulatory effects of peppermint (Mentha × piperita L.) essential oils in human leukocytes. J. Pharm. Sci. Res. 2009, 1, 33–43. [Google Scholar]
- Sanei-Dehkordi, A.; Abdollahi, A.; Safari, M.; Karami, F.; Ghaznavi, G.; Osanloo, M. Nanogels containing Foeniculum vulgare mill. and Mentha piperita L. essential oils: Mosquitoes’ repellent activity and antibacterial effect. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 4510182. [Google Scholar] [CrossRef]
- Zeljković, S.Ć.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P. Antiviral activity of selected Lamiaceae essential oils and their monoterpenes against SARS-CoV-2. Front. Pharmacol. 2022, 13, 893634. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Sancarlo, D.; Addante, F.; Scarcelli, C.; Franceschi, M. Non-steroidal anti-inflammatory drug use in the elderly. Surg. Oncol. 2010, 19, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Caroen, S.; Reid, T. What Exactly Is Inflammation (and What Is It Not?). Int. J. Mol. Sci. 2022, 23, 14905. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.A.; Perić, T.M.; Mišković, J.; Karaman, M. Antimicrobial and toxic effects of Boswellia Serrata Roxb. and Mentha piperita Linn. essential oils on vaginal inhabitants. Medicines 2022, 9, 62. [Google Scholar] [CrossRef]
- Jain, D.; Pathak, N.; Khan, S.; Raghuram, G.V.; Bhargava, A.; Samarth, R.; Mishra, P.K. Evaluation of cytotoxicity and anticarcinogenic potential of Mentha leaf extracts. Int. J. Toxicol. 2011, 30, 225–236. [Google Scholar] [CrossRef]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef]
- Berdowska, I.; Zieliński, B.; Matusiewicz, M.; Fecka, I. Modulatory impact of Lamiaceae metabolites on apoptosis of human leukemia cells. Front. Pharmacol. 2022, 13, 867709. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Rasooli, I.; Owlia, P.; Taghizadeh, M.; Astaneh, S.D. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn. Mag. 2010, 6, 147–153. [Google Scholar] [CrossRef]
- Samarth, R.M.; Panwar, M.; Kumar, M.; Kumar, A. Protective effects of Mentha piperita Linn on benzo[a]pyrene-induced lung carcinogenicity and mutagenicity in Swiss albino mice. Mutagenesis 2006, 21, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.R.; Badal, D.; Badal, P.; Khare, A.; Srrs, J.; Kumar, V. Pharmacological Action of Mentha piperita on Lipid Profile in Fructose-Fed Rats. Iran. J. Pharm. Res. 2011, 10, 843–848. [Google Scholar]
- Meamarbash, A. Instant effects of peppermint essential oil on the physiological parameters and exercise performance. Avicenna J. Phytomed. 2014, 4, 72–78. [Google Scholar]
- Javidanpour, S.; Dianat, M.; Badavi, M.; Mard, S.A. The cardioprotective effect of rosmarinic acid on acute myocardial infarction and genes involved in Ca2+ homeostasis. Free Radic. Res. 2017, 51, 911–923. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- Al-Tawarah, N.M.; Al-dmour, R.H.; Abu Hajleh, M.N.; Khleifat, K.M.; Alqaraleh, M.; Al-Saraireh, Y.M.; Jaradat, A.Q.; Al-Dujaili, E.A. Rosmarinus officinalis and Mentha piperita oils supplementation enhances memory in a rat model of scopolamine-induced alzheimer’s disease-like condition. Nutrients 2023, 15, 1547. [Google Scholar] [CrossRef]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.-J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile terpenes and brain function: Investigation of the cognitive and mood effects of Mentha × piperita L. essential oil with in vitro properties relevant to central nervous system function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef]
- Da Silva Ramos, R.; Rodrigues, A.B.; Farias, A.L.; Simões, R.C.; Pinheiro, M.T.; Ferreira, R.M.; Costa Barbosa, L.M.; Picanço Souto, R.N.; Fernandes, J.B.; da Santos, L.; et al. Chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L. (Lamiaceae). Sci. World J. 2017, 2017, 4927214. [Google Scholar] [CrossRef]
- Sydney de Sousa, A.A.; Soares, P.M.G.; Saldanha de Almeida, A.N.; Maia, A.R.; Prata de Souza, E.; Assreuy, A.M.S. Antispasmodic effect of Mentha piperita essential oil on tracheal smooth muscle of rats. J. Ethnopharmacol. 2010, 130, 433–436. [Google Scholar] [CrossRef]
- Abuirmeileh, A.; Alkhodari, A.; Alnnjeeli, A.; Talhouni, A.; Alsalahat, I.; Naddaf, A. Peppermint aqueous extract counteracts smooth muscle contraction in rat ileum. Jordan J. Pharm. Sci. 2014, 7, 88–96. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; Ramadan, L.A.; Ashry, O.M.; Kafafy, Y.A. Protective Effect of Aqueous Plant Extracts of Glycyrrhiza glabra, Melissa officinalis and Mentha × piperita Against Indomethacin Induced Gastric Ulcer in Irradiated Rats. Egypt. J. Radiat. Sci. Appl. 2004, 17, 25–45. [Google Scholar]
- Roome, T.; Qasim, M.; Farooq, A.D.; Ilyas, Q.; Aziz, S.; Ali, S.F. Antispasmodic activity and mechanism of action of polyherbal formulation DCD-684 on rabbit jejunum. Pak. J. Pharm. Sci. 2021, 34, 711–722. [Google Scholar] [PubMed]
- Khatoon, A.; Khan, F.; Ahmad, N.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Dutta, R. Silver nanoparticles from leaf extract of Mentha piperita: Eco-friendly synthesis and effect on acetylcholinesterase activity. Life Sci. 2018, 209, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, P.; Zhao, Q.; Goorani, S. Beneficial properties of the biosynthesized silver/chitosan nanoparticles mediated by Mentha piperita in rats with heart failure following myocardial infarction. Inorg. Chem. Commun. 2022, 141, 109581. [Google Scholar] [CrossRef]

| Component | Specified Components [%] | |||
|---|---|---|---|---|
| [81] | Ph. Eur. [53] | WHO [83] | ||
| Non-US Origin | US Origin | |||
| menthol | 32.0–49.0 | 36.0–46.0 | 30.0–55.0 | 30.0–55.0 |
| menthone | 13.0–28.0 | 15.0–25.0 | 14.0–32.0 | 14.0–32.0 |
| isomenthone | 2.0–8.0 | 2.0–4.5 | 1.5–10.0 | 2.0–10 |
| menthyl acetate | 2.0–8.0 | 3.0–6.5 | 2.8–10.0 | 3.0–5.0 |
| eucalyptol (1,8-cineole) | 3.0–8.0 | 4.0–6.0 | 3.5–8.0 | 6.0–14.0 |
| menthofuran | 1.0–8.0 | 1.5–6.0 | 1.0–8.0 | 1.0–9.0 |
| neomenthol | 2.0–6.0 | 2.5–4.5 | ||
| limonene | 1.0–3.0 | 1.0–2.5 | 1.0–3.5 | 1.0–5.0 |
| trans-sabinene hydrate | 0.5–2.0 | 0.5–2.3 | ||
| pulegone | 0.5–3.0 | 0.5–2.5 | 0–3.0 | 0–4.0 |
| β-caryophyllene | 1.0–3.5 | 1.0–2.5 | ||
| 3-octanol | 0.1–0.5 | 0.1–0.4 | ||
| carvone | 0–1.0 | 0–1.0 | ||
| 1,8-cineole/limonene ratio | >2.0 | |||
| Test | Tested Material, Origin | Values | Values for a Reference Substance | Reference |
|---|---|---|---|---|
| DPPH | Essential oil, Egypt | IC50 = 59.2 µg/mL | IC50 for TBHQ 29.8 µg/mL | [8] |
| DPPH | Essential oil Aqueous extract, Brazil | IC50 = 13.6 mg/mL IC50 = 12.2 mg/mL | No data | [1] |
| Pulse voltammetry | Essential oil, Brazil | Rate constants 155.9 mL/g,122.4 mL/g | [9] | |
| FRAP | Methanol/chloroform (3:1) extract, Turkey | 317.60 ± 49.32 558.33 ± 13.52 μmol trolox equivalents per one gram of dry weight | Trolox | [98] |
| TEAC | 771.58 ± 3.22 and 800 ± 10 ± 1.10 μmol trolox equivalents per one gram of dry weight | Trolox | ||
| DPPH | Essential oil, Midwest region of the USA | IC50 70.29 mg/mL | No data | [16] |
| TEAC | IC50 29.51 mg/mL | |||
| Reducing power assay | IC50 22.7 mg/mL | |||
| Lipid peroxidation assay in pig liver homogenate | Reducing lipid peroxidation in a dose-dependent manner at 1000 µg/mL; at a concentration of 2000 µg/mL there was no reducing lipid peroxidation | Malondialdehyde | ||
| Cellular antioxidant activity in jejunal epithelial cell line IPEC-J2 with DCF | Maximal inhibitory effect at a concentration of 5 µg/mL | Trolox | ||
| Intracellular antioxidant activity for glutathion (GSH) | Essential oil did not enhance GSH production. | |||
| In vivo antioxidant analysis with nematode model | The survival rate was increased at the concentrations 10, 25, 50, and 100 µg/mL; at a concentration of 200 µg/mL the survival was on the level of the control | Trolox | ||
| Test with peroxidase | Essential oil Chloroform extract Ethanolic extract Aqueous extract, Libya | 89.4% 91.2% 76.2% 69.8% | [20] | |
| DPPH | Essential oil Chloroform extract Ethanolic extract Aqueous extract | 92.6% (IC50 = 15.2 µg/mL) 91.8% 74.8% 70.3% | IC50 for BHT 6.1 µg/mL | |
| Reducing power assay, absorbance at 700 nm | Essential oil Chloroform extract Ethanolic extract Aqueous extract | 0.9 ± 0.3 0.8 ± 0.3 0.7 ± 0.1 0.4 ± 0.3 | ||
| DPPH | Ethanolic extract, Iran | IC50 13.32 µg/mL (12.12–14.64 µg/mL) | rutin, IC50 6.90 (6.61–7.20) μg/mL, | [99] |
| ABTS | IC50 153.80 µg/mL (139.90–169.00 µg/mL) | rutin, IC50 79.59 (69.73–90.86) μg/mL | ||
| DPPH | Essential oil, China | Peppermint concentrations were 200, 400, 600, 800, and 1000 mg/mL. The respective scavenging capacities ranged from 36.81% to 79.85% | BHT, from 82.36% to 93.85% | [19] |
| Table | Type of a Preparation, Doses Tested | Pharmacological Mechanisms of Action | Values | Reference |
|---|---|---|---|---|
| Carrageenan-induced paw edema test | Essential oil, oral administration 30 min before introduction of carrageenan, 2, 20, and 200 μL/kg, vehicle (isosaline NaCl 0.9%), and sodium diclofenac (50 mg/kg, orally) as the reference drug | Reducing the paw edema induced using carrageenan injection in mice | The levels of edema inhibition,12.27 ± 3.94% and 9.29 ± 3.94%, at 200 and 20 μL/kg, respectively, were comparable to the level observed using sodium diclofenac (11.43 ± 6.07%) | [60] |
| Circular excision wound model in rats followed by histological examination | The cream prepared from the essential oil (0.5% w/w) | Decreasing in unhealed wound area | Significant decrease in unhealed wound area between the 6th (1.67 ± 0.14 mm2) and 9th (0.49 ± 0.22 mm2) day of treatment in comparison with the vehicle and madecassol on 6th day (2.32 ± 0.77 mm2 and 2.23 ± 0.35 mm2, respectively) | |
| Murine macrophage cell line RAW 264.7 stimulated with LPS | The ethanolic extract of M. piperita, 25, 50, and 100 µg/mL | Reducing the LPS-induced production of NO, TNF-a, and IL-6 compared with untreated control | Reducing NO secretion in a concentration-dependent manner by 7.06, 18.85, and 41.88%, respectively. Suppression of TNF-a secretion by 20.71, 34.74, and 42.95%. Reducing IL-6 levels by 27.00, 43.71, and 51.85%. Inhibition of PGE2 production. | [12] |
| PBMCs | Essential oil, 0.01 µL/mL | Reducing IL-4 production depends on cultivar and place of growing. | [102] | |
| 5-LOX inhibition assay | Essential oil | 5-LOX inhibition | IC50 were 0.08 and 0.03 μL/mL depending on the cultivar | [80] |
| Activating antioxidant enzymes, the survival of peritoneal macrophage stimulated with lipopolysaccharide | The hydroalcoholic leaf extract of M. piperita grown in Brazil | Activating superoxide dismutase and glutathione peroxidase. Lowering the levels of H2O2. The survival was observed at the lower concentrations of the extract (1–30 µg/mL), while higher concentrations of the extract did decrease viability in the absence of lipopolysaccharides. | No data | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. https://doi.org/10.3390/molecules28217444
Hudz N, Kobylinska L, Pokajewicz K, Horčinová Sedláčková V, Fedin R, Voloshyn M, Myskiv I, Brindza J, Wieczorek PP, Lipok J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules. 2023; 28(21):7444. https://doi.org/10.3390/molecules28217444
Chicago/Turabian StyleHudz, Nataliia, Lesya Kobylinska, Katarzyna Pokajewicz, Vladimira Horčinová Sedláčková, Roman Fedin, Mariia Voloshyn, Iryna Myskiv, Ján Brindza, Piotr Paweł Wieczorek, and Jacek Lipok. 2023. "Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products" Molecules 28, no. 21: 7444. https://doi.org/10.3390/molecules28217444
APA StyleHudz, N., Kobylinska, L., Pokajewicz, K., Horčinová Sedláčková, V., Fedin, R., Voloshyn, M., Myskiv, I., Brindza, J., Wieczorek, P. P., & Lipok, J. (2023). Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules, 28(21), 7444. https://doi.org/10.3390/molecules28217444