Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential
Abstract
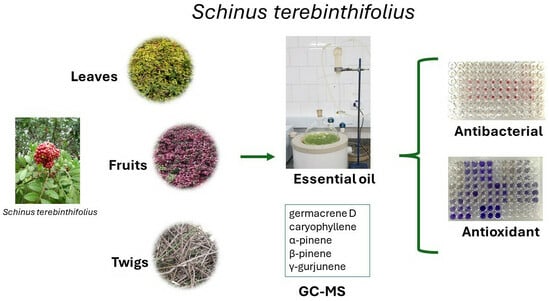
:1. Introduction
2. Results
2.1. Yield and Chemical Composition of Essential Oils
2.2. Antioxidant Activity
2.3. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Essential Oils from the Leaves, Fruits and Twigs of Schinus terebinthifolius
4.3. Analysis of the Chemical Composition of Essential Oils
4.4. Analysis of the Main Components of Essential Oils
4.5. Antioxidant Activity
4.5.1. Determination of Total Phenol Content (FT)
4.5.2. Free Radical-Scavenging Method 2,2 Diphenyl-1-picrylhydrazyl (DPPH)
4.5.3. β-Carotene/Linoleic Acid Co-Oxidation System
4.5.4. Ferrous Reduction Method (FRAP)
4.6. Antibacterial Activity
4.6.1. Microorganisms and Inoculum Preparation
4.6.2. Antibacterial Activity by Broth Microdilution Method
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Žarković, N. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Zantar, S.; Yedri, F.; Mrabet, R.; Laglaoui, A.; Bakkali, M.; Zerrouk, M.H. Effect of Thymus vulgaris and Origanum compactum essential oils on the shelf life of fresh goat cheese. J. Essent. Oil Res. 2014, 26, 76–84. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/facts-in-pictures/detail/food-safety (accessed on 2 December 2023).
- Almeida Filho, E.S.; Nader Filho, A. Occurrence of Staphylococcus aureus in cheese made in Brazil. Rev. Saúde Pública 2000, 34, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Bulhões, C.C.C.; Rossi Júnior, O.D. Occurrence of the genus Aeromonas in minas frescal cheese. Arq. Bras. Med. Vet. Zootec. 2002, 54, 320–324. [Google Scholar] [CrossRef]
- Komatsu, R.S.; Rodrigues, M.A.M.; Loreno, W.B.N.; Santos, K.A. Ocorrência de Staphylococcus coagulase positiva em queijos Minas frescal produzidos em Uberlândia-MG. Biosci. J. 2010, 26, 316–321. [Google Scholar]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Andrade, M.A.; Cardoso, M.G.; Batista, L.R.; Mallet, A.C.T.; Machado, S.M.F. Essential oils of Cinnamomum zeylanicum, Cymbopogon nardus and Zingiber officinale: Composition, antioxidant and antibacterial activities. Rev. Ciênc. Agron. 2012, 43, 399–408. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards, and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Orth, A.I. Fenologia reprodutiva, morfologia e biologia floral de Schinus terebinthifolius Raddi (Anacardiaceae), em restinga da ilha de Santa Catarina, Brasil. Biotemas 2004, 17, 67–89. [Google Scholar]
- Cesário, L.F.; Gaglianone, M.C. Pollinators of Schinus terebinthifolius Raddi (Anacardiaceae) in vegetational formations of restinga in northern Rio de Janeiro state. Biosci. J. 2013, 29, 458–467. [Google Scholar]
- Oliveira, L.F.M.; Oliveira Junior, L.F.G.; Santos, M.C.; Narain, N.; Leite Neto, M.T.S. Distillation time and volatile profile of the essential oil of Brazilian pepper (Schinus terebinthifolius) in Sergipe, Brazil. Rev. Bras. Plantas Med. 2014, 16, 243–249. [Google Scholar] [CrossRef]
- Ferreira-Filho, P.J.; Pinã-Rodrigues, F.C.M.; Silva, J.M.S.; Guerreiro, J.C.; Ghiotto, T.C.; Piotrowski, I.; Dias, L.P.; Wilcken, C.F.; Zanuncio, J. The exotic wasp Megastigmus transvaalensis (Hymenoptera: Torymidae): First record and damage on the Brazilian peppertree, Schinus terebinthifolius drupes, in São Paulo, Brazil. An. Acad. Bras. Cienc. 2015, 87, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.G.; Melo, A.G.N.; Aragão, C.F.S.; Raffin, F.N.; Moura, T.F.A.L. Schinus terebinthifolius Raddi: Chemical composition, biological properties and toxicity. Rev. Bras. Plantas Med. 2013, 15, 158–169. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde. Agência Nacional de Vigilância Sanitária. In Monografia da Espécie Schinus terebinthifolius Raddi (Aroeira-da-praia); Anvisa: Brasília, Brazil, 2014. [Google Scholar]
- Estevão, L.R.M.; Simões, R.S.; Cassini-Vieira, P.; Canesso, M.C.C.; Barcelos, L.D.S.; Rachid, M.A.; Câmara, C.A.G.D.; Evêncio-Neto, J. Schinus terebinthifolius raddi (aroeira) leaves oil attenuates inflammatory responses in cutaneous wound healing in mice. Acta Cir. Bras. 2017, 32, 726–735. [Google Scholar] [CrossRef]
- Rosas, E.C.; Correa, L.B.; Henriques, M.G. Antiinflammatory Properties of Schinus terebinthifolius and Its Use in Arthritic Condition. In Book Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Ronald Ross Watson, R.R., Victor, R., Preedy, V.R., Eds.; Academic Press: London, UK, 2019; pp. 489–505. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations World Health Organization. Discussion Paper on the Grouping of Spices and Culinary Herbs. In Codex Committee on Spices and Culinary Herbs; FAO/WHO: Rome, Italy, 2015. [Google Scholar]
- Ruas, E.A.; Ruas, C.F.; Medri, P.S.; Medi, C.; Medri, M.E.; Bianchini, E.; Pimenta, J.A.; Rodrigues, L.A.; Ruas, P.M. Anatomy and genetic diversity of two populations of Schinus terebinthifolius (Anacardiaceae) from the Tibagi River basin in Paraná, Brazil. Genet. Mol. Res. 2011, 10, 526–536. [Google Scholar] [CrossRef]
- Dannenberg, G.S.; Funck, G.D.; Silva, W.P.; Fiorentini, A.M. Essential oil from pink pepper (Schinus terebinthifolius Raddi): Chemical composition, antibacterial activity and mechanism of action. Food Control 2019, 95, 115–120. [Google Scholar] [CrossRef]
- Bortolucci, W.C.; Oliveira, H.L.M.; Silva, E.S.; Campo, C.F.A.A.; Gonçalves, J.E.; Piau Junior, R.; Colauto, N.B.; Linde, G.A.; Gazim, Z.C. Schinus terebinthifolius essential oil and fractions in the control of Aedes aegypti. Biosci. J. 2019, 35, 391–404. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Behiry, S.I.; Ali, H.M.; El-Hefny, M.; Salem, M.Z.M.; Ashmawy, N.A. Phytochemicals from branches of the oily liquid extract of P. halepensis and essential oil of S. terebinthifolius and their potential antifungal activity. Processes 2020, 8, 330. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Ennigrou, A.; Casabianca, H.; Vulliet, E.; Hanchi, B.; Hosni, K. Assessing the fatty acid, essential oil composition, their radical scavenging and antibacterial activities of Schinus terebinthifolius Raddi leaves and twigs. J. Food Sci. Technol. 2018, 55, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Belhoussaine, O.; El Kourchi, C.; Harhar, H.; Bouyahya, A.; El Yadini, A.; Fozia, F.; Alotaibi, A.; Ullah, R.; Tabyaoui, M. Chemical composition, antioxidant, insecticidal activity, and comparative analysis of essential oils of leaves and fruits of Schinus molle and Schinus terebinthifolius. Evid. Based Complement. Altern. Med. 2022, 30, 4288890. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2010; p. 991. [Google Scholar]
- Nemeth, E.; Bernath, J. Biological activities of yarrow species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013; pp. 1–43. [Google Scholar]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Sahin, F.; Gulluce, M.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M.; Agar, G.; Ozer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Dannenberg, G.D.; Funck, G.D.; Mattei, F.J.; Silva, W.P.; Fiorentini, Â.M. Antimicrobial and antioxidant activity of essential oil from pink pepper tree (Schinus terebinthifolius Raddi) in vitro and in cheese experimentally contaminated with Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2016, 36, 120–127. [Google Scholar] [CrossRef]
- Carneiro, M.J.; Pinheiro, G.P.; Baseggio, A.M.; Maróstica-Júnior, M.R.; Sawaya, A.C.H.F. Chemical Composition and Antioxidant Activity of Essential Oil from Male and Female Schinus terebinthifolius. Pharmacogn. Res. 2023, 15, 484–491. [Google Scholar] [CrossRef]
- Sokmen, A.; Abdel-Baki, A.A.S.; Al-Malki, E.S.; Al-Quraishy, S.; Abdel-Hallem, H.B. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. J. King Saud. Univ. Sci. 2020, 32, 2377–2382. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Yunfeng, Z.; Lin, L.; Lan, S.; Zhou Lidong, Z.; Yang, X. In comparison with vitamin C and butylated hydroxytoluene, the antioxidant capacity of aqueous extracts from buds and flowers of Lonicera japonica. Thunb. J. Tradit. Chin. Med. 2018, 38, 373–379. [Google Scholar] [CrossRef]
- Kulisic, T.; Radanic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef]
- Yunes, R.A.; Cechinel Filho, V. Química de Produtos Naturais, Novos Fármacos e a Moderna Farmacognosia, 2nd ed.; Univale: Itajaí, Brasil, 2009; pp. 219–256. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Sousa, E.L.F.; Arias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial activity and Time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicano, T.M.; Saturnino, C.; Malzert-Freon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. J. 2019, 10, 4280–4290. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural food colorants and preservatives: A review, a demand, and a challenge. J. Agric. Food. Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Gundimeda, U.; Naidu, A.N.; Krishnaswamy, K. Dietary intake of nitrate in India. J. Food Compos. Anal. 1993, 6, 242–249. [Google Scholar] [CrossRef]
- Ezeagu, I.E. Nitrate and nitrite contents in ogi and the changes occurring during storage. Food Chem. 1996, 56, 77–79. [Google Scholar] [CrossRef]
- Zhang, D.S.T.; Lin, X. Accumulation of nitrate in vegetables and its possible implications to human health. Agric. Sci. China 2007, 6, 1246–1255. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol. Lett. 2011, 200, 107–108. [Google Scholar] [CrossRef]
- Santos, M.R.A.; Lima, R.A.; Silva, A.G.; Lima, D.K.S.; Sallet, L.A.P.; Teixeira, C.A.D.; Facundo, V.A. Chemical composition and insecticidal activity of the essential oil of Schinus terebinthifolius Raddi (Anacardiaceae) on coffee berry borer (Hypothenemus hampei) Ferrari. Rev. Bras. Plantas Med. 2013, 15, 757–762. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017; p. 698. [Google Scholar]
- Moita Neto, J.M.; Moita, G.C. An introduction analysis exploratory multivariate date. Quim. Nova 1998, 21, 467–469. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.; Hair, J. Análise Multivariada de Dados, 1st ed.; Bookman: Porto Alegre, Brasil, 2005; p. 688. [Google Scholar]
- Ferré, L. Selection of components in principal component analysis: A comparison of methods. Comput. Stat. Data Anal. 1995, 19, 669–682. [Google Scholar] [CrossRef]
- Camacho, J.; Picó, J.; Ferrer, A. Data understanding with PCA: Structural and variance information plots. Chemometr. Intell. Lab. Syst. 2010, 100, 48–56. [Google Scholar] [CrossRef]
- Statsoft Inc. Statistica for Windows (Computer Program Manual). Available online: http://www.statsoft.com/ (accessed on 20 July 2018).
- Swain, T.; Hillis, W.E. The Phenolic Constituents of Prunus domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Sousa De Sá, P.G.; Guimarães, A.L.; Oliveira, A.P.; Filho, J.A.S.; Fontana, A.P.; Damasceno, P.K.F.; Branco, C.R.C.; Branco, A.; Almeida, J.R.G.S. Total phenols, total flavonoids and antioxidant activity of Selaginella convoluta (Arn.) Spring (Selaginellaceae). Rev. Ciênc. Farm. Básica Apl. 2012, 33, 561–566. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Jiménez, J.P.; Calixto, F.D.S. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pela Captura do Radical Livre DPPH; Embrapa: Brasília, Brazil, 2007; Volume 127, pp. 1–4. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Mancini Filho, J.; Moreira, A.V.B. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas no Sistema β-Caroteno/Ácido Linoleico; Embrapa: Brasília, Brazil, 2006; Volume 126, pp. 1–4. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Jiménez, J.P.; Calixto, F.D.S. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pelo Método de Redução do Ferro (FRAP); Embrapa: Brasília, Brazil, 2006; Volume 125, pp. 1–4. [Google Scholar]
- Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically; Approved Standard M7-A10; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]

| Peak | RT 1 | Compound | RI 2 | RI | RA % 3 | ||
|---|---|---|---|---|---|---|---|
| Leaves | Fruits | Twigs | |||||
| 1 | 5.833 | α-pinene | 934 | 932 | 11.6 | 17.16 | 2.99 |
| 2 | 7.13 | β-pinene | 976 | 974 | 5.68 | 43.34 | 5.60 |
| 3 | 7.13 | α-phellandrene | 1003 | 1002 | 0.61 | 0.85 | 0.44 |
| 4 | 8.523 | 3-carene | 1009 | 1008 | 0.79 | - | 0.54 |
| 5 | 8.803 | α-terpinene | 1014 | 1014 | - | - | 0.17 |
| 6 | 9.144 | p-cymene | 1019 | 1020 | - | - | 0.43 |
| 7 | 9.32 | D-limonene | 1018 | 1024 | - | 1.92 | 1.50 |
| 8 | 9.321 | β-phellandrene | 1023 | 1025 | 0.43 | - | 0.66 |
| 9 | 9.324 | eucalyptol | 1025 | 1026 | 1.49 | - | - |
| 10 | 10.274 | β-cis-ocimene | 1039 | 1032 | - | - | 0.26 |
| 11 | 10.717 | γ-terpinene | 1048 | 1054 | - | - | 0.40 |
| 12 | 17.051 | terpinen-4-ol | 1178 | 1174 | - | - | 1.18 |
| 13 | 17.882 | α-terpineol | 1185 | 1186 | - | - | 0.42 |
| 14 | 26.657 | δ-eIemene | 1330 | 1335 | 0.71 | - | - |
| 15 | 27.442 | α-cubebene | 1343 | 1345 | - | - | 1.31 |
| 16 | 28.963 | α-copaene | 1373 | 1374 | 5.25 | - | 5.73 |
| 17 | 29.88 | β-cubebene | 1383 | 1387 | 1.55 | - | 1.94 |
| 18 | 30.03 | β-elemene | 1390 | 1389 | 4.57 | - | 1.44 |
| 19 | 31.549 | α-gurjunene | 1409 | 1409 | 2.15 | 2.12 | 0.82 |
| 20 | 31.549 | caryophyllene | 1418 | 1417 | 15.97 | 3.12 | 11.73 |
| 21 | 32.621 | (-)-aristolene | 1427 | 1428 | - | - | 0.40 |
| 22 | 33.155 | α-himachalene | 1446 | 1449 | - | - | 1.42 |
| 23 | 33.493 | α-humulene | 1452 | 1452 | 1.91 | - | 1.99 |
| 24 | 34.913 | E-β-farnesene | 1454 | 1454 | - | - | 1.31 |
| 25 | 34.212 | allo-aromadendrene | 1459 | 1458 | 2.25 | - | 1.49 |
| 26 | 34.928 | γ-gurjunene | 1475 | 1475 | 16.85 | 3.15 | - |
| 27 | 35.241 | γ-muurolene | 1476 | 1478 | - | - | 1.27 |
| 28 | 35.45 | germacrene D | 1481 | 1484 | 12.04 | 15.78 | 20.41 |
| 29 | 36.435 | valencene | 1495 | 1496 | - | - | 6.38 |
| 30 | 36.569 | α-selinene | 1497 | 1498 | 1.33 | - | - |
| 31 | 37.148 | α-muurolene | 1499 | 1500 | - | - | 0.63 |
| 32 | 37.262 | δ-amorphene | 1509 | 1511 | 4.94 | 3.21 | 0.77 |
| 33 | 37.811 | δ-cadinene | 1503 | 1522 | - | - | 5.59 |
| 34 | 38.212 | cadina-1,4-diene | 1510 | 1524 | - | - | 0.33 |
| 35 | 39.29 | elemol | 1530 | 1548 | - | 2.7 | - |
| 36 | 40.628 | spathulenol | 1573 | 1577 | 3.75 | 1.21 | 5.47 |
| 37 | 40.92 | caryophyllene oxide | 1578 | 1582 | 1.99 | 0.77 | 4.83 |
| 38 | 41.041 | globulol | 1589 | 1590 | 1.11 | - | - |
| 39 | 42.167 | viridiflorol | 1590 | 1592 | - | - | 0.61 |
| 40 | 43.577 | epicubenol | 1603 | 1617 | - | - | 0.66 |
| 41 | 44.316 | τ-cadinol | 1618 | 1625 | 1.02 | - | 2.17 |
| 42 | 44.632 | torreyol | 1624 | 1632 | - | - | 0.39 |
| 43 | 45.046 | τ-muurulol | 1638 | 1640 | - | - | 1.08 |
| 44 | 69.658 | mandenol | 2151 | 2159 | - | 2.02 | - |
| Total Identified | 97.99 | 97.35 | 94.76 | ||||
| monoterpenes hydrocarbons | 19.11 | 63.27 | 12.99 | ||||
| oxygenated monoterpenes | 1.49 | - | 1.6 | ||||
| sesquiterpenes hydrocarbons | 69.52 | 30.08 | 64.96 | ||||
| oxygenated sesquiterpenes | 7.87 | 1.98 | 15.21 | ||||
| diterpene oxygenated | - | 2.02 | - | ||||
| Samples | DPPH | FRAP | Total Phenolics |
|---|---|---|---|
| IC50 (mg mL−1) | (µM Ferrous Sulphate mg−1) | (µg AGE mg−1) | |
| Leaves | 5.368 ± 0.132 b | 0.434 ± 0.005 b | 23.66 ± 1.60 b |
| Fruits | 14.760 ± 0.108 d | 0.438 ± 0.002 b | 24.79 ± 0.37 a |
| Twigs | 12.690 ± 0.483 c | 0.437 ± 0.004 b | 19.17 ± 0.72 c |
| Quercetin | 0.01 ± 0.01 a | - | - |
| Trolox | - | 9.175 ± 0.01 a | - |
| Samples | Concentrations (mg mL−1) | |||
|---|---|---|---|---|
| 1 | 0.75 | 0.5 | 0.25 | |
| Leave | 63.65 ± 1.38 dC | 55.23 ± 2.25 cB | 50.63 ± 2.75 bB | 44.93 ± 2.85 aA |
| Fruits | 61.52 ± 1.13 dB | 54.60 ± 1.27 cA | 49.69 ± 2.45 aA | 54.09 ± 2.94 bC |
| Twigs | 40.75 ± 2.10 aA | 58.76 ± 0.71 dC | 51.62 ± 2.53 cC | 47.95 ± 1.24 bB |
| Bacteria | Leaves | Fruits | Twigs | Sodium Nitrite |
|---|---|---|---|---|
| (mg mL−1) | (mg mL−1) | (mg mL−1) | (mg mL−1) | |
| MIC | MIC | MIC | MIC | |
| MBC | MBC | MBC | MBC | |
| Staphylococcus aureus | 1.25 ± 0.00 b | 10.00 ± 0.01 d | 2.50 ± 0.00 c | 5.00 ± 0.00 c |
| 5.00 ± 0.01 b | 20.00 ± 0.01 d | 10.00 ± 0.00 c | >20.00 ±0.00 d | |
| Escherichia coli | 0.62 ± 0.00 b | 10.00 ± 0.00 d | 20.00 ±0.002 e | 5.00 ± 0.00 c |
| 20.00 ± 0.00 b | >20.00 ± 0.00 b | >20.00 ±0.00 b | >20.00 ± 0.00 b | |
| Bacillus cereus | 0.62 ± 0.00 b | 10.00 ± 0.00 d | 10.00 ± 0.00 d | 5.00 ± 0.00 c |
| 10.00 ± 0.01 b | 10.00 ± 0.00 b | 20.00 ± 0.00 c | >20.00 ± 0.00 c | |
| Salmonella Typhi | 2.50 ± 0.00 b | 10.00 ± 0.01 d | >20.00 ± 0.02 e | 5.00 ± 0.00 c |
| 20.00 ± 0.00 b | >20.00 ± 0.00 b | >20.00 ± 0.00 b | >20.00 ± 0.01 b | |
| Pseudomonas aeruginosa | 2.50 ± 0.00 b | 5.00 ± 0.00 c | 20.00 ±0.00 d | 5.00 ± 0.00 c |
| 2.50 ± 0.00 b | 10.00 ± 0.00 c | 20.00 ±0.00 d | >20.00 ± 0.00 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, K.C.; Franciscato, L.M.S.S.; Mendes, S.S.; Barizon, F.M.A.; Gonçalves, D.D.; Barbosa, L.N.; Faria, M.G.I.; Valle, J.S.; Casalvara, R.F.A.; Gonçalves, J.E.; et al. Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules 2024, 29, 469. https://doi.org/10.3390/molecules29020469
Oliveira KC, Franciscato LMSS, Mendes SS, Barizon FMA, Gonçalves DD, Barbosa LN, Faria MGI, Valle JS, Casalvara RFA, Gonçalves JE, et al. Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules. 2024; 29(2):469. https://doi.org/10.3390/molecules29020469
Chicago/Turabian StyleOliveira, Kátia C., Lidaiane M. S. S. Franciscato, Suelen S. Mendes, Francielly M. A. Barizon, Daniela D. Gonçalves, Lidiane N. Barbosa, Maria G. I. Faria, Juliana S. Valle, Rhaira F. A. Casalvara, José E. Gonçalves, and et al. 2024. "Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential" Molecules 29, no. 2: 469. https://doi.org/10.3390/molecules29020469
APA StyleOliveira, K. C., Franciscato, L. M. S. S., Mendes, S. S., Barizon, F. M. A., Gonçalves, D. D., Barbosa, L. N., Faria, M. G. I., Valle, J. S., Casalvara, R. F. A., Gonçalves, J. E., Gazim, Z. C., & Ruiz, S. P. (2024). Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules, 29(2), 469. https://doi.org/10.3390/molecules29020469