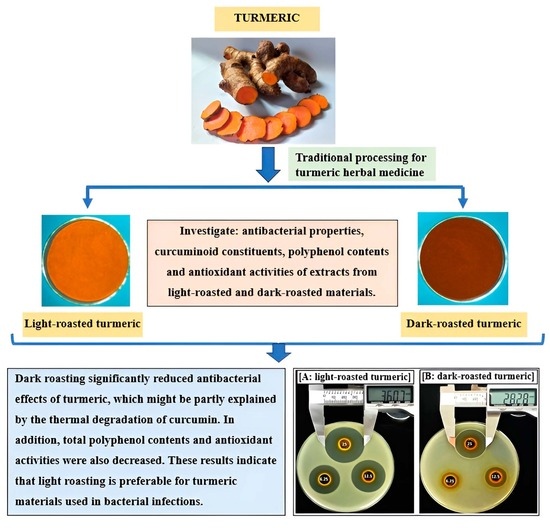
Effects of Roasting Conditions on Antibacterial Properties of Vietnamese Turmeric (Curcuma longa) Rhizomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Roasting on Curcuminoid Contents of Turmeric
2.2. Effects of Roasting on Antibacterial Effects of Turmeric
2.2.1. Effects of Roasting on MIC Values of Turmeric Ethanol Extracts
2.2.2. Effects of Roasting on Inhibitory Zones of Turmeric Ethanol Extracts
2.3. Effects of Roasting on Total Polyphenol and Antioxidant Activities of Turmeric
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Reagents and Bacterial Strains
3.3. HPLC Analysis of Curcuminoids
3.4. Evaluation of Antibacterial Effects of the Extracts
3.5. Determination of Total Polyphenol and Antioxidant Activities
3.6. Statistical Analysis
4. Conclusions
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Do, T.L. (Ed.) Curcuma longa L. In Common Medicinal Plants and Traditional Therapies in Vietnam, 12th ed.; Vietnam Ministry of Health; Medical Publishing House: Hanoi, Vietnam, 1999; pp. 227–230. [Google Scholar]
- Vietnam Ministry of Health. Rhizoma Curcuma longae. In Vietnamese Pharmacopoeia, 5th ed.; Medical Publishing House: Hanoi, Vietnam, 2019; pp. 1264–1265. [Google Scholar]
- Website for Vietnamese Medicinal Search: Tracuuduoclieu.vn. Golden Turmeric. Available online: https://tracuuduoclieu.vn/nghe-vang.html (accessed on 10 September 2023).
- Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine. Chapter 2: Processing techniques of traditional medicine. In Text-Book of Pharmaceutics and Pharmaceutical Technology (Internal Circulated Documents for Education of Veterinary Medicine), 1st ed.; Vietnam National University of Agriculture Publishing House: Hanoi, Vietnam, 2022; pp. 45–52. [Google Scholar]
- Ajatta, M.A.; Akinola, S.A.; Otolowo, D.T.; Awolu, O.O.; Omoba, O.S.; Osundahunsi, O.F. Effect of roasting on the phytochemical properties of three varieties of marble vine (Dioclea reflexa) using response surface methodology. Prev. Nutr. Food Sci. 2019, 24, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Xu, F.; Li, Y.L.; Shang, M.Y.; Makino, T.; Cai, S.Q. Comparison of chemical constituents among licorice, roasted licorice, and roasted licorice with honey. J. Nat. Med. 2018, 72, 80–95. [Google Scholar] [CrossRef]
- Suresh, D.; Gurudutt, K.N.; Srinivasan, K. Degradation of bioactive spice compound: Curcumin during domestic cooking. Eur. Food Res. Technol. 2009, 228, 807–812. [Google Scholar] [CrossRef]
- Suresh, D.; Manjunatha, H.; Srinivasan, K. Effect of heat processing of spices on the concentrations of their bioactive principles: Turmeric (Curcuma longa), red pepper (Capsicum annuum) and black pepper (Piper nigrum). J. Food Compos. Anal. 2007, 20, 346–351. [Google Scholar] [CrossRef]
- Sun, J.L.; Ji, H.F.; Shen, L. Impact of cooking on the antioxidant activity of spice turmeric. Food Nutr. Res. 2019, 31, 63. [Google Scholar] [CrossRef] [PubMed]
- Dahmke, I.N.; Boettcher, S.P.; Groh, M.; Mahlknecht, U. Cooking enhances curcumin anti-cancerogenic activity through pyrolytic formation of “deketene curcumin”. Food Chem. 2014, 151, 514–519. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Wu, J.N.; Tu, Q.K.; Xiang, X.L.; Shi, Q.X.; Chen, G.Y.; Dai, M.X.; Zhang, L.J.; Yang, M.; Song, C.W.; Huang, R.Z.; et al. Changes in curcuminoids between crude and processed turmeric based on UPLC-QTOF-MS/MS combining with multivariate statistical analysis. Chin. J. Anal. Chem. 2022, 50, 100108. [Google Scholar] [CrossRef]
- Naidu, M.M.; Shyamala, B.N.; Manjunatha, J.R.; Sulochanamma, G.; Srinivas, P. Simple HPLC method for resolution of curcuminoids with antioxidant potential. J. Food Sci. 2009, 74, C312–C318. [Google Scholar] [CrossRef]
- Dandekar, P.P.; Patravale, V.B. Development and validation of a stability-indicating LC method for curcumin. Chromatographia 2009, 69, 871–877. [Google Scholar] [CrossRef]
- Peram, M.R.; Jalalpure, S.S.; Palkar, M.B.; Diwan, P.V. Stability studies of pure and mixture form of curcuminoids by reverse phase-HPLC method under various experimental stress conditions. Food Sci. Biotechnol. 2017, 26, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Lawhavinit, O.A.; Kongkathip, N.; Kongkathip, B. Antimicrobial activity of curcuminoids from Curcuma longa L. on pathogenic bacteria of shrimp and chicken. Kasetsart J. Nat. Sci. 2010, 44, 364–371. [Google Scholar]
- Wang, Y.; Lu, Z.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [PubMed]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Zandi, K.; Fouladvand, M.; Ramedani, E. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3823–3835. [Google Scholar]
- Sandikci Altunatmaz, S.; Yilmaz Aksu, F.; Issa, G.; Basaran Kahraman, B.; Dulger Altiner, D.; Buyukunal, S.K. Antimicrobial effects of curcumin against L. monocytogenes, S. aureus, S. typhimurium and E. coli O157:H7 pathogens in minced meat. Vet. Med. Czech. 2016, 61, 256–262. [Google Scholar] [CrossRef]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Sivasothy, Y.; Sulaiman, S.F.; Ooi, K.L.; Ibrahim, H.; Awang, K. Antioxidant and antibacterial activities of flavonoids and currcuminoids from Zingiber spectabile Griff. Food Control 2013, 30, 714–720. [Google Scholar]
- Zermeño-Ruiz, M.; Rangel-Castañeda, I.A.; Suárez-Rico, D.O.; Hernández-Hernández, L.; Cortés-Zárate, R.; Hernández-Hernández, J.M.; Camargo-Hernández, G.; Castillo-Romero, A. Curcumin stimulates the overexpression of virulence factors in Salmonella enterica Serovar Typhimurium: In vitro and animal model studies. Antibiotics 2022, 11, 1230. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Signh, R.P.; Jain, D.A. Evaluation of antimicrobial activity of curcuminoids isolated from turmeric. Int. J. Pharm. Biol. Sci. 2012, 3, 1368–1376. [Google Scholar]
- Afzal, A.; Oriqat, G.; Akram Khan, M.; Jose, J.; Afzal, M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J. Biol. Active Prod. Nat. 2013, 3, 1–55. [Google Scholar] [CrossRef]
- Wilson, B.; Abraham, G.; Manju, V.S.; Mathew, M.; Vimala, B.; Sundaresan, S.; Nambisan, B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005, 99, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Akter, J.; Islam, M.Z.; Hossain, M.A.; Kawabata, S.; Takara, K.; Nguyen, H.T.T.; Hou, D.X.; Miyamoto, A. Endothelium-independent and calcium channel-dependent relaxation of the porcine cerebral artery by different species and strains of turmeric. J. Tradit. Complement. Med. 2018, 9, 297–303. [Google Scholar] [CrossRef]
- Akter, J.; Amzad Hossain, M.; Sano, A.; Takara, K.; Islam, M.Z.; Hou, D.X. Antifungal activity of various species and strains of turmeric (Curcuma spp.) against Fusarium Solani Sensu Lato. Pharm. Chem. J. 2018, 52, 320–325. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules 2019, 9, 13. [Google Scholar]
- Sasaki, Y.; Goto, H.; Tohda, C. Effects of Curcuma drugs on vasomotion in isolated rat aorta. Biol. Pharm. Bull. 2003, 26, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Tonnesen, H. Studies on curcumin and curcuminoids XV. catalytic effect of demethoxy and bisdemethoxycurcumin on the peroxidation of linoleic acid by 15-lipoxygenase. Int. J. Pharm. 1989, 51, 179–181. [Google Scholar] [CrossRef]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; Akao, N.; Kondo, K.; Tsuda, Y. Nematocidal activity of turmeric: Synergistic action of curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Sakib Hossen, M.; Fuad Hossain, M.; Afroz, R.; Gan, S.H.; Ibrahim Khalil, M.; Karim, N. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Wangsawat, N.; Nahar, L.; Sarker, S.D.; Phosri, C.; Evans, A.R.; Whalley, A.J.S.; Choowongkomon, K.; Suwannasai, N. Antioxidant activity and cytotoxicity against cancer cell lines of the extracts from novel Xylaria species associated with termite nests and LC-MS analysis. Antioxidants 2021, 10, 1557. [Google Scholar] [CrossRef]
- Akter, J.; Amzad Hossain, M.; Takara, K.; Zahorul Islam, M.; Hou, D.X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): Isolation of active compounds. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.M.; Zeb, A.; Mahmood, M.; Abbas, S.R.; Ahmad, Z.; Iqbal, N. Phytochemical analysis and biological analysis and biological activities of ethanolic extract of Curcuma longa rhizome. Braz. J. Biol. 2021, 81, 37–740. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Ban, I.; Lee, H.; Baik, M.Y.; Kim, W. Puffing as a novel process to enhance the antioxidant and anti-inflammatory properties of Curcuma longa L. (Turmeric). Antioxidants 2019, 8, 506. [Google Scholar] [CrossRef]
- Kim, H.; Ban, I.; Choi, Y.; Yu, S.; Youn, S.J.; Baik, M.Y.; Lee, H.; Kim, W. Puffing of turmeric (Curcuma longa L.) enhances its anti-inflammatory effects by upregulating macrophage oxidative phosphorylation. Antioxidants 2020, 9, 931. [Google Scholar] [CrossRef]
- Vietnam Ministry of Health. General Guidance on the Traditional Processing of Medicinal Plants. Circular Number 30/2017/TT-BYT, issued on 7 November 2017. Available online: https://thuvienphapluat.vn/van-ban/The-thao-Y-te/Thong-tu-30-2017-TT-BYT-huong-dan-phuong-phap-che-bien-cac-vi-thuoc-co-truyen-358031.aspx (accessed on 10 September 2023).
- Nguyen, H.T.T.; Nguyen, H.T.; Islam, M.Z.; Obi, T.; Pothinuch, P.; Zar, P.P.K.; Hou, D.X.; Van Nguyen, T.; Nguyen, T.M.; Van Dao, C.; et al. Pharmacological characteristics of Artemisia vulgaris L. in isolated porcine basilar artery. J. Ethnopharmacol. 2016, 182, 16–26. [Google Scholar] [CrossRef]
- Vietnam National Institute for Food Control. List of Accredited Tests. Number 38: “General Instructions for the Determination of Curcuminoid Content by HPLC Method” (Code: NIFC.05.M.132). Documentary Number: 894.2020/QĐ-VPCNCL, issued on 17 November 2020. Available online: http://www.boa.gov.vn/sites/default/files/203tt1120kngvpt.pdf (accessed on 3 September 2023).
- M100-S17; Performance Standards for Antimicrobials Susceptibility Testing (Suppl. 17). Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2007.
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Suda, I.; Oki, T.; Nishiba, Y.; Masuda, M.; Kobayashi, M.; Nagai, S.; Hiyane, R.; Miyashige, T. Polyphenol contents and radical scavenging activity of extracts from fruits and vegetables cultivated in Okinawa, Japan. Nippon Shokuhin Kagaku Kogaku Kaishi 2005, 52, 462–471. [Google Scholar] [CrossRef]
- Masuda, T.; Oyama, Y.; Inaba, Y.; Toi, Y.; Arata, T.; Takeda, Y.; Nakamoto, K.; Kuninaga, H.; Nishizato, S.; Nonaka, A. Antioxidant related activities of ethanol extracts from edible and medicinal plants cultivated in Okinawa, Japan. Nippon Shokuhin Kagaku Kogaku Kaishi 2002, 49, 652–661. [Google Scholar] [CrossRef]
- Wetwitayaklung, P.; Phaechamud, T.; Limmatvapirat, C.; Keokitichai, S. The study of antioxidant activities of edible flower extracts. Acta Hortic. 2008, 786, 185–192. [Google Scholar] [CrossRef]




| Material | Curcumin | Demethoxy-Curcumin | Bisdemethoxy-Curcumin | Total Curcuminoid |
|---|---|---|---|---|
| Non-roasted turmeric | 4.611 a ± 0.042 | 2.228 ± 0.110 | 1.146 ± 0.071 | 7.985 a ± 0.207 |
| Light-roasted turmeric | 4.635 a ± 0.073 | 2.157 ± 0.127 | 1.154 ± 0.043 | 7.945 a ± 0.064 |
| Dark-roasted turmeric | 3.060 b ± 0.030 | 2.168 ± 0.225 | 1.149 ± 0.028 | 6.376 b ± 0.197 |
| Tested Material | Gram (+) | Gram (−) | ||||
|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli 25922 | E. coli 85922 | S. Typhimurium | P. aeruginosa | |
| Non-roasted turmeric | 62.5 | 125 | 250 | 250 | 250 | 250 |
| Light-roasted turmeric | 62.5 | 125 | 250 | 250 | 250 | 250 |
| Dark-roasted turmeric | 125 | 250 | 500 | 500 | 500 | 500 |
| Curcumin | 125 | 125 | 250 | 250 | 250 | 250 |
| Bacterium | Material | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|---|
| Gram (+) | B. subtilis | Extract | 50 mg/mL | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL |
| Non–roasted | 24.25 a* ± 0.14 | 21.29 a ± 0.57 | 17.73 a ± 1.10 | 8.41 a ± 0.75 | ||
| Light–roasted | 24.18 a ± 0.73 | 20.91 a ± 0.76 | 17.66 a ± 0.79 | 8.44 a ± 1.28 | ||
| Dark–roasted | 14.91 b ± 1.33 | 14.23 b ± 1.06 | 10.89 b ± 0.67 | 3.44 b ± 0.46 | ||
| Curcumin | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | 3.13 mg/mL | ||
| 10.42 ± 0.70 | 7.80 ± 0.23 | 5.04 ± 0.64 | 3.07 ± 0.26 | |||
| S. aureus | Extract | 50 mg/mL | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | |
| Non–roasted | 31.65 a* ± 1.09 | 25.89 a ± 0.16 | 14.85 a ± 0.72 | 10.06 a ± 0.90 | ||
| Light–roasted | 31.19 a ± 0.43 | 25.40 a ± 0.69 | 15.21 a ± 1.97 | 9.91 a ± 1.06 | ||
| Dark–roasted | 20.91 b ± 0.85 | 17.57 b ± 0.67 | 7.22 b ± 0.65 | 3.71 b ± 0.28 | ||
| Curcumin | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | 3.13 mg/mL | ||
| 12.58 ± 1.39 | 9.64 ± 0.76 | 6.61 ± 1.09 | 3.06 ± 0.06 | |||
| Gram (−) | E. coli ATCC 25922 | Extract | 50 mg/mL | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL |
| Non–roasted | 20.36 a* ± 1.63 | 10.79 a ± 0.33 | 7.95 a ± 0.39 | 5.00 ± 0.91 | ||
| Light–roasted | 20.55 a ± 1.23 | 10.12 a ± 1.29 | 7.78 a ± 0.86 | 5.03 ± 1.16 | ||
| Dark–roasted | 14.34 b ± 0.69 | 7.39 b ± 0.85 | 4.70 b ± 0.47 | - | ||
| Curcumin | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | 3.13 mg/mL | ||
| 7.99 ± 0.12 | 5.66 ± 0.78 | 3.74 ± 1.03 | - | |||
| E. coli ATCC 85922 | Extract | 50 mg/mL | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | |
| Non–roasted | 16.78 a* ± 2.26 | 12.56 a ± 2.05 | 9.66 a ± 1.17 | 5.57 ± 0.79 | ||
| Light–roasted | 17.11 a ± 0.47 | 12.33 a ± 3.82 | 8.99 a ± 0.95 | 5.66 ± 1.84 | ||
| Dark–roasted | 11.68 b ± 0.33 | 7.66 b ± 0.84 | 5.25 b ± 0.58 | - | ||
| Curcumin | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | 3.13 mg/mL | ||
| 7.81 ± 0.24 | 5.78 ± 0.58 | 3.45 ± 0.71 | - | |||
| P. aeruginosa | Extract | 50 mg/mL | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | |
| Non–roasted | 12.51 a* ± 0.83 | 9.66 a ± 0.69 | 7.02 a ± 0.32 | 3.63 ± 0.13 | ||
| Light–roasted | 11.64 a ± 0.85 | 9.66 a ± 1.74 | 7.01 a ± 0.69 | 3.75 ± 0.52 | ||
| Dark–roasted | 9.79 b ± 0.34 | 7.46 b ± 0.47 | 4.05 b ± 0.27 | - | ||
| Curcumin | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | 3.13 mg/mL | ||
| 7.77 ± 0.50 | 3.76 ± 0.52 | - | - | |||
| S. Typhimurium | Extract | 50 mg/mL | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | |
| Non–roasted | 9.85 a* ± 0.42 | 7.30 a ± 0.52 | 6.27 ± 0.31 | 5.40 ± 0.45 | ||
| Light–roasted | 10.12 a ± 1.18 | 7.77 a ± 0.67 | 6.44 ± 0.68 | 5.17 ± 0.81 | ||
| Dark–roasted | 6.15 b ± 0.86 | 4.74 b ± 1.20 | - | - | ||
| Curcumin | 25 mg/mL | 12.5 mg/mL | 6.25 mg/mL | 3.13 mg/mL | ||
| 4.54 ± 0.56 | 3.07 ± 0.13 | - | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Wu, S.; Ootawa, T.; Nguyen, H.C.; Tran, H.T.; Pothinuch, P.; Pham, H.T.T.; Do, A.T.H.; Hoang, H.T.; Islam, M.Z.; et al. Effects of Roasting Conditions on Antibacterial Properties of Vietnamese Turmeric (Curcuma longa) Rhizomes. Molecules 2023, 28, 7242. https://doi.org/10.3390/molecules28217242
Nguyen HT, Wu S, Ootawa T, Nguyen HC, Tran HT, Pothinuch P, Pham HTT, Do ATH, Hoang HT, Islam MZ, et al. Effects of Roasting Conditions on Antibacterial Properties of Vietnamese Turmeric (Curcuma longa) Rhizomes. Molecules. 2023; 28(21):7242. https://doi.org/10.3390/molecules28217242
Chicago/Turabian StyleNguyen, Hai Thanh, Siyuan Wu, Tomoki Ootawa, Hieu Chi Nguyen, Hong Thi Tran, Pitchaya Pothinuch, Hang Thi Thu Pham, Anh Thi Hong Do, Hao Thanh Hoang, Md. Zahorul Islam, and et al. 2023. "Effects of Roasting Conditions on Antibacterial Properties of Vietnamese Turmeric (Curcuma longa) Rhizomes" Molecules 28, no. 21: 7242. https://doi.org/10.3390/molecules28217242
APA StyleNguyen, H. T., Wu, S., Ootawa, T., Nguyen, H. C., Tran, H. T., Pothinuch, P., Pham, H. T. T., Do, A. T. H., Hoang, H. T., Islam, M. Z., Miyamoto, A., & Nguyen, H. T. T. (2023). Effects of Roasting Conditions on Antibacterial Properties of Vietnamese Turmeric (Curcuma longa) Rhizomes. Molecules, 28(21), 7242. https://doi.org/10.3390/molecules28217242