Study on Purification, Identification and Antioxidant of Flavonoids Extracted from Perilla leaves
Abstract
:1. Introduction
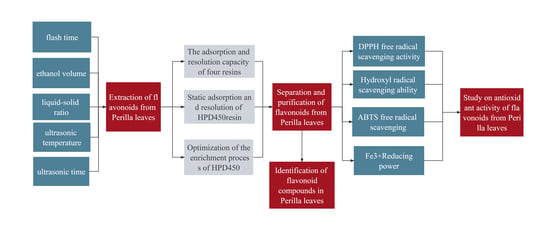
2. Results and Analysis
2.1. Optimization of Extraction Process of Flavonoids from Perilla leaves
2.1.1. Single Factor Test Results
Effect of Liquid–Solid Ratio on Extraction Rate of Flavonoids from Perilla leaves
Effect of Ethanol Volume Fraction on Extraction Yield of Total Flavonoids from Perilla leaves
Effect of Ultrasonic Temperature on Extraction of Flavonoids from Perilla leaves
Effect of Ultrasonic Time on Extraction of Flavonoids from Perilla leaves
Effect of Flash Time on Extraction of Flavonoids from Perilla leaves
2.1.2. Box-Behnken Test Results
2.2. Separation and Purification of Flavonoids from Perilla leaves
2.2.1. Static Adsorption and Resolution of Resin
2.2.2. Separation and Purification of Flavonoids from Perilla leaves
2.2.3. Optimization of the Enrichment Process of HPD450 Macroporous Resin
The Influence of Sample Amount on the Resolution Rate of HPD450 Macroporous Resin
Influence of Eluent Concentration on Resolution Rate of HPD450 Macroporous Resin
Effect of Eluate Volume on the Concentration of Flavonoids in Perilla leaves
2.3. Identification of Flavonoid Compounds in Perilla leaves
2.4. Study on Antioxidant Activity of Flavonoids from Perilla leaves
2.4.1. DPPH Free Radical Scavenging Activity of Perilla leaf Flavonoids
2.4.2. Hydroxyl Radical Scavenging Ability of Perilla leaf Flavonoids
2.4.3. ABTS Free Radical Scavenging Ability of Perilla leaf Flavonoids
2.4.4. Effect of Perilla leaf Flavonoids on Fe3+ Reducing Power
3. Materials and Methods
3.1. Materials and Instruments
3.2. Experimental Methods
3.2.1. Extraction Technology of Flavonoids from Perilla leaves
3.2.2. Optimization of Extraction Process of Flavonoids from Perilla leaves
3.2.3. Isolation and Purification of Flavonoids from Perilla leaves
Pretreatment of Resin
Resin Screening
Dynamic Curve of HPD450 Resin
Optimization of Enrichment Process for HPD450 Resin
3.2.4. Identification of Flavonoids in Perilla leaves
3.2.5. Study on Antioxidant Activity
DPPH Free Radical Scavenging Experiment
Determination of Hydroxyl Radical Scavenging Ability
ABTS+• Scavenging Effect of Free Radicals
Determination of Reducing Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity–ScienceDirect. Ind. Crops Prod. 2020, 143, 111908. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, D.; Sun, W.; Zhang, Y.J.; Shang, Z.W.; Chen, S.L. Medicinal plant DNA marker assisted breeding (Ⅱ) the assistant identification of SNPs assisted identification and breeding research of high yield Perilla frutescens new variety. Zhongguo Zhong Yao Za Zhi 2017, 42, 1668–1672. [Google Scholar] [PubMed]
- Wang, Z.-X.; Lin, Q.-Q.; Tu, Z.-C.; Zhang, L. The influence of in vitro gastrointestinal digestion on the Perilla frutescens leaf extract: Changes in the active compounds and bioactivities. J. Food Biochem. 2020, 44, e13530. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.J.; Kim, K.C.; Kim, H.; Kim, J.-S.; Hyun, T.K. Variability of Polyphenolic Compounds and Biological Activities among Perilla frutescens var. crispa Genotypes. Horticulturae 2021, 7, 404. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Li, D.Y.; Sun, J.; Cheng, J.M.; Chai, C.; Zhang, L.; Peng, G.P. Comprehensive Comparison of Two Color Varieties of Perillae Folium Using Rapid Resolution Liquid Chromatography Coupled with Quadruple-Time-of-Flight Mass Spectrometry (RRLC-Q/TOF-MS)-Based Metabolic Profile and in Vivo/in Vitro Anti-Oxidative Activity. J. Agric. Food Chem. 2020, 68, 14684–14697. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Guo, K.; Liu, L.; Tian, W.; Xie, X.; Wen, S.; Wen, C. Integrated transcriptomic and metabolomic data reveal the flavonoid biosynthesis metabolic pathway in Perilla frutescens (L.) leaves. Sci. Rep. 2020, 10, 16207. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Wu, Y.; Shen, X.; Xu, S.; Guo, Z.; Zhang, R.; Xing, D. The Physiologic Activity and Mechanism of Quercetin-Like Natural Plant Flavonoids. Curr. Pharm. Biotechnol. 2020, 21, 654–658. [Google Scholar] [CrossRef]
- Cazarolli, H.L.; Zanatta, L.; Alberton, H.E.; Bonorino Figueiredo, R.M.S.; Folador, P.; Damazio Guollo, R.; Pizzolatti, G.M.; Barreto, S.M.F.R. Flavonoids: Prospective Drug Candidates. Mini-Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Li, Z.-N.; Zhang, J.-M.; Hou, X.-Y.; Yeh, M.S.; Ming, X. Recent Developments of Flavonoids with Various Activities. Curr. Top. Med. Chem. 2022, 22, 305–329. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamamoto, Y.; Yoshinaka, N.; Namba, M.; Matsuo, H.; Okuyama, T.; Yoshigai, E.; Okumura, T.; Nishizawa, M.; Ikeya, Y. A new flavanone and other flavonoids from green Perilla leaf extract inhibit nitric oxide production in interleukin 1 beta-treated hepatocytes. Biosci. Biotechnol. Biochem. 2015, 79, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kaewseejan, N.; Sutthikhum, V.; Siriamornpun, S. Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J. Funct. Foods 2015, 12, 120–128. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.-J.; Zheng, X.; Gao, M.-Z.; Meng, Y.; Wang, W. Efficient extraction of flavonoids from Flos Sophorae Immaturus by tailored and sustainable deep eutectic solvent as green extraction media. J. Pharm. Biomed. Anal. 2018, 170, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, G.; Li, G.; Yang, Z. Optimization of extraction of total flavonoids from alfalfa leaves and its antioxidant activity by response surface methodology. J. Chin. Food Sci. 2016, 16, 145–152. [Google Scholar]
- Hou, M.; Hu, W.; Wang, A.; Xiu, Z.; Shi, Y.; Hao, K.; Sun, X.; Cao, D.; Lu, R.; Sun, J. Ultrasound-Assisted Extraction of Total Flavonoids from Pteris cretica L. Process Optimization, HPLC Analysis, and Evaluation of Antioxidant Activity. Antioxidants 2019, 8, 425. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Akhtar, H.M.S.; Chen, D.; Wan, P.; Chen, G.; Zeng, X. Extraction, purification by macrospores resin and in vitro antioxidant activity of flavonoids from Moringa oliefera leaves. S. Afr. J. Bot. 2019, 124, 270–279. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Liao, F.; Zhang, M. Studies of optimum technology on flash type extraction methods of total flavonoids from the fruits of Pholidota chinensis Lindl. Lishizhen Med. Mater. Medica Res. Huangshi Inst. Technol. 2016, 27, 317–319. [Google Scholar]
- Wang, Q.-Z.; Yuan, T.-F.; Liu, L.-L.; Wang, H.; Guo, J. Optimization of Flash Extraction of Total Flavonoids from Leaves of Podocarpus macrophyllus by Response Surface Method and Their Antioxidant Activity. Fine Chem. 2018, 35, 65–71. [Google Scholar]
- Sheng, Z.-L.; Wan, P.-F.; Dong, C.-L.; Li, Y.H. Optimization of total flavonoids content extracted from Flos Populi using response surface methodology. Ind. Crops Prod. 2013, 43, 778–786. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Wang, D.; Niu, Y. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochem. 2022, 90, 106190. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, M.; Sun-Water House, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Q.; Zhang, D.; Shi, Y.; Jiang, C.; Shi, X. Preliminary separation and purification of resveratrol from extract of peanut (Arachis hypogaea) sprouts by macroporous adsorption resins. Food Chem. 2014, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Xia, L.; Lei, F.H. Application of macroporous adsorption resin in the separation and purification of natural products. Chem. Technol. Dev. 2021, 50, 29–34. [Google Scholar]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Feng, J.; He, X.; Zhou, S.; Peng, F.; Liu, J.; Hao, L.; Li, H.; Ao, G. Preparative separation of crocins and geniposide simultaneously from gardenia fruits using macroporous resin and reversed-phase chromatography. J. Sep. Sci. 2014, 37, 314–322. [Google Scholar] [CrossRef]
- Zhu, J.b.; Cui, W.b.; Xiao, W.; Ding, Y.; Huang, W.; Tu, P.; Wang, Y. Isolation and enrichment of Ginkgo biloba extract by a continuous chromatography system. J. Sep. Sci. 2018, 41, 2432–2440. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pilar, R.; Fernando, G.; Adrián, M.; García-Carmona, F.; López-Nicolás, J.M. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS+, and FRAP Techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar]
- Yin, N.; Wang, Y.; Ren, X.; Zhao, Y.; Liu, N.; An, X.; Qi, J. Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo. Fermentation 2022, 8, 306. [Google Scholar] [CrossRef]
- Yu, J.; Lou, Q.; Zheng, X.; Cui, Z.; Fu, J. Sequential Combination of Microwave- and Ultrasound-Assisted Extraction of Total Flavonoids from Osmanthus fragrans Lour. Flowers. Molecules 2017, 22, 2216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Pang, X.; Hua, P.; Gao, X.; Li, Q.; Li, Z. Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM). Molecules 2019, 24, 3461. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yang, G.; Zhang, J.; Li, J.; Bai, B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules 2020, 25, 1767. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
















| The No. | Liquid/Material Ratio (mL/g) | Ethanol Volume Fraction (%) | Ultrasonic Temperature (°C) | Flavonoids Extraction Yield (mg/g) |
|---|---|---|---|---|
| 1 | 20 | 50 | 70 | 9.04 |
| 2 | 15 | 60 | 70 | 6.77 |
| 3 | 20 | 60 | 60 | 9.82 |
| 4 | 15 | 70 | 60 | 6.57 |
| 5 | 20 | 70 | 50 | 8.99 |
| 6 | 25 | 60 | 70 | 9.35 |
| 7 | 20 | 60 | 60 | 9.76 |
| 8 | 20 | 60 | 60 | 9.78 |
| 9 | 20 | 70 | 70 | 9.19 |
| 10 | 15 | 50 | 60 | 6.75 |
| 11 | 25 | 50 | 60 | 9.03 |
| 12 | 25 | 60 | 50 | 9.08 |
| 13 | 20 | 60 | 60 | 9.81 |
| 14 | 25 | 70 | 60 | 9.43 |
| 15 | 20 | 60 | 60 | 9.79 |
| 16 | 15 | 60 | 50 | 6.66 |
| 17 | 20 | 50 | 50 | 9.06 |
| Sources of Variance | Sum of Squares | Degrees of Freedom | The Mean Square | The F Value | p Values | Significant |
|---|---|---|---|---|---|---|
| model | 23.90 | 9 | 2.66 | 1526.18 | <0.0001 | ** |
| A | 12.85 | 1 | 12.85 | 7386.47 | <0.0001 | |
| B | 0.011 | 1 | 0.011 | 6.47 | 0.0385 | |
| C | 0.039 | 1 | 0.039 | 22.53 | 0.0021 | |
| AB | 0.084 | 1 | 0.084 | 48.33 | 0.0002 | |
| AC | 6.4 × 10−3 | 1 | 6.4 × 10−3 | 3.68 | 0.0966 | |
| BC | 0.012 | 1 | 0.012 | 6.95 | 0.0336 | |
| A2 | 9.17 | 1 | 9.17 | 5271.81 | <0.0001 | |
| B2 | 0.58 | 1 | 0.58 | 333.07 | <0.0001 | |
| C2 | 0.52 | 1 | 0.52 | 298.13 | <0.0001 | |
| residual | 0.012 | 7 | 1.74 × 10−3 | |||
| Loss of quasi | 9.9 × 10−3 | 3 | 3.3 × 10−3 | 5.79 | 0.0614 | |
| Pure error | 2.28 × 10−3 | 4 | 5.7 × 10−4 | |||
| The sum of the | 23.91 | 16 |
| Factors | The Level of Factors | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Liquid/material ratio (mL/g) | 15:1 | Those days | 25:1 |
| Ethanol volume fraction (%) | 50 | 60 | 70 |
| Ultrasonic temperature (°C) | 50 | 60 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lin, J.; Bai, B.; Bo, T.; He, Y.; Fan, S.; Zhang, J. Study on Purification, Identification and Antioxidant of Flavonoids Extracted from Perilla leaves. Molecules 2023, 28, 7273. https://doi.org/10.3390/molecules28217273
Li H, Lin J, Bai B, Bo T, He Y, Fan S, Zhang J. Study on Purification, Identification and Antioxidant of Flavonoids Extracted from Perilla leaves. Molecules. 2023; 28(21):7273. https://doi.org/10.3390/molecules28217273
Chicago/Turabian StyleLi, Hui, Jiayu Lin, Baoqing Bai, Tao Bo, Yufei He, Shanhong Fan, and Jinhua Zhang. 2023. "Study on Purification, Identification and Antioxidant of Flavonoids Extracted from Perilla leaves" Molecules 28, no. 21: 7273. https://doi.org/10.3390/molecules28217273
APA StyleLi, H., Lin, J., Bai, B., Bo, T., He, Y., Fan, S., & Zhang, J. (2023). Study on Purification, Identification and Antioxidant of Flavonoids Extracted from Perilla leaves. Molecules, 28(21), 7273. https://doi.org/10.3390/molecules28217273