Synthesis, Spectroscopic Characterization, Catalytic and Biological Activity of Oxidovanadium(V) Complexes with Chiral Tetradentate Schiff Bases
Abstract
:1. Introduction
2. Results and Discussion
2.1. IR Spectra
2.2. Electronic and Circular Dichroism Spectra
2.3. NMR Measurements
2.4. Catalytic Activity Studies
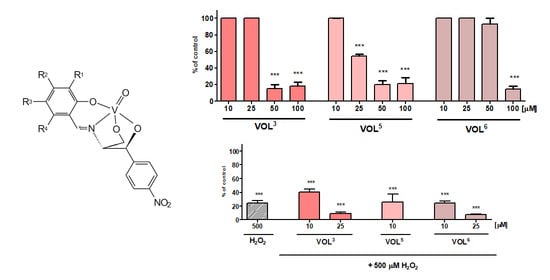
2.5. Biological Studies
3. Materials and Methods
3.1. Measurements
3.2. Synthesis of Oxidovanadium(V) Complexes
3.3. Catalytic Activity
3.4. Biological Activity
3.4.1. The Cell Culture
3.4.2. Treatments
3.4.3. MTT Assay
3.4.4. Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carter-Franklin, J.N.; Parrish, J.D.; Tschirret-Guth, R.A.; Little, R.D.; Butler, A. Vanadium haloperoxidase-catalyzed bromination and cyclization of terpenes. J. Am. Chem. Soc. 2003, 125, 3688–3689. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D.; Santoni, G.; Licini, G.M.; Schulzke, C.; Meier, B. The medicinal and catalytic potential of model complexes of vanadate-dependent haloperoxidases. Coord. Chem. Rev. 2003, 237, 53–63. [Google Scholar] [CrossRef]
- Littlechild, J.A.; Garcia-Rodriguez, E. Structural studies on the dodecameric vanadium bromoperoxidase from Corallina species. Coord. Chem. Rev. 2003, 237, 65–76. [Google Scholar] [CrossRef]
- Eady, R.R. Current status of structure function relationships of vanadium nitrogenase. Coord. Chem. Rev. 2003, 237, 23–30. [Google Scholar] [CrossRef]
- Rehder, D. Bioinorganic Vanadium Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Weyand, M.; Hecht, H.J.; Kiess, M.; Liaud, M.F.; Vilter, H.; Schomburg, D. X-ray structure determination of a vanadium-dependent haloperoxidase from Ascophyllum nodosum at 2.0 Å resolution. J. Mol. Biol. 1999, 293, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Ligtenbarg, A.G.J.; Hage, R.; Feringa, B.L. Catalytic oxidations by vanadium complexes. Coord. Chem. Rev. 2003, 237, 89–101. [Google Scholar] [CrossRef]
- Bolm, C. Vanadium-catalyzed asymmetric oxidations. Coord. Chem. Rev. 2003, 237, 245–256. [Google Scholar] [CrossRef]
- Hashmi, K.; Satya; Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal applications of vanadium complexes with Schiff bases. J. Trace Elem. Med. Biol. 2023, 79, 127245. [Google Scholar] [CrossRef] [PubMed]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic applications of vanadium: A mechanistic perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged chiral catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Pradeep, C.P.; Das, S.K. Coordination and supramolecular aspects of the metal complexes of chiral N-salicyl-β-amino alcohol Schiff base ligands: Towards understanding the roles of weak interactions in their catalytic reactions. Coord. Chem. Rev. 2013, 257, 1699–1715. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Kuo, Y.-P.; Gau, H.-M. Synthesis, characterization, and structures of oxovanadium(V) complexes of Schiff bases of β-amino alcohols as tunable catalysts for the asymmetric oxidation of organic sulfides and asymmetric alkynylation of aldehydes. Dalton Trans. 2007, 2007, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, H.; Weng, W.; Lin, W.; Gao, Y.; Huang, X.; Zhao, Y. Substituent effects and mechanism elucidation of enantioselective sulfoxidation catalyzed by vanadium Schiff base complexes. New J. Chem. 2005, 29, 1125–1127. [Google Scholar] [CrossRef]
- Wojaczyńska, E.; Wojaczyński, J. Enantioselective synthesis of sulfoxides: 2000–2009. Chem. Rev. 2010, 110, 4303–4356. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J. Stereoselective syntheses of functionalized cyclic ethers via (Schiff-base) vanadium(V)-catalyzed oxidations. Pure Appl. Chem. 2005, 77, 1559–1574. [Google Scholar] [CrossRef]
- Hartung, J.; Drees, S.; Greb, M.; Schmidt, P.; Svoboda, I.; Fuess, H.; Murso, A.; Stalke, D. (Schiff-base)vanadium(V) complex-catalyzed oxidations of substituted bis(homoallylic) alcohols—Stereoselective synthesis of functionalized tetrahydrofurans. Eur. J. Org. Chem. 2003, 2003, 2388–2408. [Google Scholar] [CrossRef]
- Cordelle, C.; Agustin, D.; Daran, J.-C.; Poli, R. Oxo-bridged bis oxo-vanadium(V) complexes with tridentate Schiff base ligands (VOL)2O (L = SAE, SAMP, SAP): Synthesis, structure and epoxidation catalysis under solvent-free. Inorg. Chim. Acta 2010, 364, 144–149. [Google Scholar] [CrossRef]
- Romanowski, G.; Kira, J.; Wera, M. Vanadium(V) complexes with chiral tridentate Schiff base ligands derived from 1S,2R(+)-2-amino-1,2-diphenylethanol and with acetohydroxamate co-ligand: Synthesis, characterization and catalytic activity in the oxidation of prochiral sulfides and olefins. J. Mol. Catal. A Chem. 2014, 381, 148–160. [Google Scholar] [CrossRef]
- Moschona, F.; Savvopoulou, I.; Tsitopoulou, M.; Tataraki, D.; Rassias, G. Epoxide syntheses and ring-opening reactions in drug development. Catalysts 2020, 10, 1117. [Google Scholar] [CrossRef]
- Benjamin, S.L.; Burgess, K. Metal-catalyzed epoxidations of alkenes with hydrogen peroxide. Chem. Rev. 2003, 103, 2457–2474. [Google Scholar]
- Costa Pessoa, J.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in biological action: Chemical, pharmacological aspects, and metabolic implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed]
- Jakusch, T.; Kiss, T. In vitro study of the antidiabetic behavior of vanadium compounds. Coord. Chem. Rev. 2017, 351, 118–126. [Google Scholar] [CrossRef]
- Kim, A.T.; Kim, D.O. Anti-inflammatory effects of vanadium-binding protein from Halocynthia roretzi in LPS-stimulated RAW264.7 macrophages through NF-κB and MAPK pathways. Int. J. Biol. Macromol. 2019, 133, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D. Potentiality of vanadium compounds as anti-parasitic agents. Coord. Chem. Rev. 2011, 255, 2193–2203. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Nareetsile, F.; Matshwele, J.T.; Ndlovu, S.; Ngaski, M. Transition metal complexes with HIV/AIDS inhibitory properties. Chem. Rev. Lett. 2020, 3, 140–160. [Google Scholar]
- Semiz, S. Vanadium as potential therapeutic agent for COVID-19: A focus on its antiviral, antiinflamatory, and antihyperglycemic effects. J. Trace Elem. Med. Biol. 2022, 69, 126887. [Google Scholar] [CrossRef]
- Correia, I.; Adão, P.; Roy, S.; Wahba, M.; Matos, C.; Maurya, M.R.; Marques, F.; Pavan, F.R.; Leite, C.Q.F.; Avecilla, F.; et al. Hydroxyquinoline derived vanadium(IV and V) and copper(II) complexes as potential anti-tuberculosis and anti-tumor agents. J. Inorg. Biochem. 2014, 141, 83–93. [Google Scholar] [CrossRef]
- Tsave, O.; Petanidis, S.; Kioseoglou, E.; Yavropoulou, M.P.; Yovos, J.G.; Anestakis, D.; Tsepa, A.; Salifoglou, A. Role of vanadium in cellular and molecular immunology: Association with immune-related inflammation and pharmacotoxicology mechanisms. Oxid. Med. Cell. Longev. 2016, 2016, 4013639. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Inkielewicz-Stępniak, I.; Pranczk, J.; Żamojć, K.; Zięba, P.; Tesmar, A.; Jacewicz, D.; Ossowski, T.; Chmurzyński, L. Physicochemical properties of ternary oxovanadium(IV) complexes with oxydiacetate and 1,10-phenanthroline or 2,2′-bipyridine. Cytoprotective activity in hippocampal neuronal HT22 cells. Biometals 2015, 28, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Tesmar, A.; Inkielewicz-Stępniak, I.; Sikorski, A.; Wyrzykowski, D.; Jacewicz, D.; Zięba, P.; Pranczk, J.; Ossowski, T.; Chmurzyński, L. Structure, physicochemical and biological properties of newcomplex salt of aqua-(nitrilotriacetato-N,O,O′,O″)-oxidovanadium(IV) anion with 1,10-phenanthrolinium cation. J. Inorg. Biochem. 2015, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.; Jaeckel, M.; Lewerenz, J.; Noack, R.; Pouya, A.; Schacht, T.; Hoffmann, C.; Winter, J.; Schweiger, S.; Schäfer, M.K.E.; et al. Mitochondrial function and energy metabolism in neuronal HT22 cells resistant to oxidative stress. Br. J. Pharmacol. 2014, 171, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, G.; Lis, T. Chiral oxidovanadium(V) complexes with tridentate Schiff bases derived from S(+)-2-amino-1-propanol: Synthesis, structure, characterization and catalytic activity. Inorg. Chim. Acta 2013, 394, 627–634. [Google Scholar] [CrossRef]
- Romanowski, G.; Kira, J. Oxidovanadium(V) complexes with chiral tridentate Schiff bases derived from R(−)-phenylglycinol: Synthesis, spectroscopic characterization and catalytic activity in the oxidation of sulfides and styrene. Polyhedron 2013, 53, 172–178. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gosh, T. Synthesis, spectral and electrochemical studies of alkoxo-bonded mixed-ligand oxovanadium(IV) and oxovanadium(V) complexes incorporating tridentate ONO donor azophenolalcoholate/aldiminealcoholates. Transit. Met. Chem. 2002, 27, 89–94. [Google Scholar] [CrossRef]
- Romanowski, G. Synthesis, characterization and catalytic activity in the oxidation of sulfides and styrene of vanadium(V) complexes with tridentate Schiff base ligands. J. Mol. Catal. A Chem. 2013, 368–369, 137–144. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Jafarpour, M.; Raissi, H.; Alipour, M.; Stoeckli-Evans, H. Economical oxygenation of olefins and sulfides catalyzed by new molybdenum(VI) tridentate Schiff base complexes: Synthesis and crystal structure. Z. Anorg. Allg. Chem. 2012, 638, 1023–1030. [Google Scholar] [CrossRef]
- Romanowski, G.; Kira, J.; Wera, M. Five- and six-coordinate vanadium(V) complexes with tridentate Schiff base ligands derived from S(+)-isoleucinol: Synthesis, characterization and catalytic activity in the oxidation of sulfides and olefins. Polyhedron 2014, 67, 529–539. [Google Scholar] [CrossRef]
- Hulea, V.; Dumitriu, E. Styrene oxidation with H2O2 over Ti-containing molecular sieves with MFI, BEA and MCM-41 topologies. Appl. Catal. A Gen. 2004, 277, 99–106. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, A.; Ebel, M.; Rehder, D. Synthesis, characterization, reactivity, and catalytic potential of model vanadium(IV,V) complexes with benzimidazole-derived ONN donor ligands. Inorg. Chem. 2006, 45, 5924–5937. [Google Scholar] [CrossRef] [PubMed]
- Maurya, M.R.; Bisht, M.; Chaudhary, N.; Avecilla, F.; Kumar, U.; Hsu, H.-F. Synthesis, structural characterization, encapsulation in zeolite Y and catalytic activity of an oxidovanadium(V) complex with a tribasic pentadentate ligand. Polyhedron 2013, 54, 180–188. [Google Scholar] [CrossRef]
- Monfared, H.H.; Bikas, R.; Mayer, P. Homogeneous green catalysts for olefin oxidation by mono oxovanadium(V) complexes of hydrazone Schiff base ligands. Inorg. Chim. Acta 2010, 363, 2574–2583. [Google Scholar] [CrossRef]
- Monfared, H.H.; Kheirabadi, S.; Lalami, N.A.; Mayer, P. Dioxo- and oxovanadium(V) complexes of biomimetic hydrazone ONO and NNS donor ligands: Synthesis, crystal structure and catalytic reactivity. Polyhedron 2011, 30, 1375–1384. [Google Scholar] [CrossRef]
- Koola, J.D.; Kochi, J.K. Cobalt-catalyzed epoxidation of olefins. Dual pathways for oxygen atom transfer. J. Org. Chem. 1987, 52, 4545–4553. [Google Scholar] [CrossRef]
- Karman, M.; Romanowski, G. Synthesis, spectroscopic characterization and catalytic activity of cis-dioxidomolybdenum(VI) complexes with chiral tetradentate Schiff bases. Appl. Organomet. Chem. 2020, 34, e5968. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef]
- Kothandan, S.; Sheela, A. DNA interaction and cytotoxic studies on mono/di-oxo and peroxo-vanadium (V) complexes—A review. Mini-Rev. Med. Chem. 2021, 21, 1909–1924. [Google Scholar] [CrossRef]
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Metallo-Drugs: Development and Action of Anticancer Agents; Chapter 9; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; De Gruyter: Berlin, Germany, 2018; pp. 251–274. [Google Scholar]








| Entry | Catalyst | Yield (%) | Oxidant | Styrene Oxide (%) | Benzaldehyde (%) |
|---|---|---|---|---|---|
| 1 | VOL1 | 14 | H2O2 | 7 | 93 |
| 2 | VOL2 | 20 | H2O2 | 11 | 89 |
| 3 | VOL3 | 16 | H2O2 | 5 | 95 |
| 4 | VOL4 | 22 | H2O2 | 8 | 92 |
| 5 | VOL5 | 21 | H2O2 | 10 | 90 |
| 6 | VOL6 | 22 | H2O2 | 6 | 94 |
| 7 | VOL7 | 23 | H2O2 | 12 | 88 |
| 8 | VOL8 | 20 | H2O2 | 8 | 92 |
| 9 | VOL9 | 18 | H2O2 | 11 | 89 |
| 10 | VOL10 | 26 | H2O2 | 7 | 93 |
| 11 | VOL1 | 84 | TBHP | 31 | 69 |
| 12 | VOL2 | 74 | TBHP | 17 | 83 |
| 13 | VOL3 | 87 | TBHP | 29 | 71 |
| 14 | VOL4 | 85 | TBHP | 28 | 72 |
| 15 | VOL5 | 63 | TBHP | 39 | 61 |
| 16 | VOL6 | 67 | TBHP | 38 | 62 |
| 17 | VOL7 | 61 | TBHP | 32 | 68 |
| 18 | VOL8 | 81 | TBHP | 27 | 73 |
| 19 | VOL9 | 84 | TBHP | 37 | 63 |
| 20 | VOL10 | 68 | TBHP | 22 | 78 |
| Entry | Catalyst | Yield (%) | Oxidant | Epoxide (%) | Alcohol (%) | Ketone (%) |
|---|---|---|---|---|---|---|
| 1 | VOL1 | 64 | H2O2 | 45 | 38 | 17 |
| 2 | VOL2 | 45 | H2O2 | 60 | 40 | - |
| 3 | VOL3 | 66 | H2O2 | 56 | 34 | 10 |
| 4 | VOL4 | 41 | H2O2 | 58 | 33 | 9 |
| 5 | VOL5 | 45 | H2O2 | 70 | 14 | 16 |
| 6 | VOL6 | 67 | H2O2 | 48 | 43 | 9 |
| 7 | VOL7 | 69 | H2O2 | 52 | 44 | 4 |
| 8 | VOL8 | 65 | H2O2 | 48 | 27 | 25 |
| 9 | VOL9 | 59 | H2O2 | 42 | 41 | 17 |
| 10 | VOL10 | 66 | H2O2 | 58 | 42 | - |
| 11 | VOL1 | 85 | TBHP | 26 | 32 | 42 |
| 12 | VOL2 | 88 | TBHP | 29 | 39 | 32 |
| 13 | VOL3 | 80 | TBHP | 37 | 26 | 37 |
| 14 | VOL4 | 87 | TBHP | 20 | 42 | 28 |
| 15 | VOL5 | 82 | TBHP | 17 | 46 | 37 |
| 16 | VOL6 | 84 | TBHP | 19 | 45 | 36 |
| 17 | VOL7 | 83 | TBHP | 11 | 56 | 33 |
| 18 | VOL8 | 84 | TBHP | 13 | 57 | 30 |
| 19 | VOL9 | 85 | TBHP | 22 | 40 | 38 |
| 20 | VOL10 | 82 | TBHP | 24 | 48 | 28 |
| Entry | Catalyst | Yield (%) | Oxidant | Epoxide (%) | Diopoxide (%) |
|---|---|---|---|---|---|
| 1 | VOL1 | 23 | H2O2 | 84 | 16 |
| 2 | VOL3 | 25 | H2O2 | 76 | 24 |
| 3 | VOL9 | 19 | H2O2 | 82 | 18 |
| 4 | VOL1 | 59 | TBHP | 69 | 31 |
| 5 | VOL2 | 71 | TBHP | 50 | 50 |
| 6 | VOL3 | 67 | TBHP | 64 | 36 |
| 7 | VOL4 | 66 | TBHP | 65 | 35 |
| 8 | VOL5 | 62 | TBHP | 65 | 35 |
| 9 | VOL6 | 76 | TBHP | 57 | 43 |
| 10 | VOL7 | 72 | TBHP | 62 | 38 |
| 11 | VOL8 | 63 | TBHP | 69 | 31 |
| 12 | VOL9 | 55 | TBHP | 72 | 28 |
| 13 | VOL10 | 58 | TBHP | 63 | 37 |
| Entry | Catalyst | Yield (%) | Oxidant | (−)-α-Pinene Oxide (%) |
|---|---|---|---|---|
| 1 | VOL1 | 14 | H2O2 | 86 |
| 2 | VOL3 | 18 | H2O2 | 88 |
| 3 | VOL9 | 15 | H2O2 | 81 |
| 4 | VOL1 | 51 | TBHP | 61 |
| 5 | VOL2 | 59 | TBHP | 73 |
| 6 | VOL3 | 64 | TBHP | 59 |
| 7 | VOL4 | 55 | TBHP | 63 |
| 8 | VOL5 | 54 | TBHP | 67 |
| 9 | VOL6 | 45 | TBHP | 66 |
| 10 | VOL7 | 48 | TBHP | 49 |
| 11 | VOL8 | 66 | TBHP | 62 |
| 12 | VOL9 | 58 | TBHP | 59 |
| 13 | VOL10 | 62 | TBHP | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowski, G.; Budka, J.; Inkielewicz-Stepniak, I. Synthesis, Spectroscopic Characterization, Catalytic and Biological Activity of Oxidovanadium(V) Complexes with Chiral Tetradentate Schiff Bases. Molecules 2023, 28, 7408. https://doi.org/10.3390/molecules28217408
Romanowski G, Budka J, Inkielewicz-Stepniak I. Synthesis, Spectroscopic Characterization, Catalytic and Biological Activity of Oxidovanadium(V) Complexes with Chiral Tetradentate Schiff Bases. Molecules. 2023; 28(21):7408. https://doi.org/10.3390/molecules28217408
Chicago/Turabian StyleRomanowski, Grzegorz, Justyna Budka, and Iwona Inkielewicz-Stepniak. 2023. "Synthesis, Spectroscopic Characterization, Catalytic and Biological Activity of Oxidovanadium(V) Complexes with Chiral Tetradentate Schiff Bases" Molecules 28, no. 21: 7408. https://doi.org/10.3390/molecules28217408
APA StyleRomanowski, G., Budka, J., & Inkielewicz-Stepniak, I. (2023). Synthesis, Spectroscopic Characterization, Catalytic and Biological Activity of Oxidovanadium(V) Complexes with Chiral Tetradentate Schiff Bases. Molecules, 28(21), 7408. https://doi.org/10.3390/molecules28217408