1. Introduction
Diabetes is a complex metabolic disease associated with disorders of glucose or lipid metabolism, with chronic hyperglycemia as the main feature. The number of adults with diabetes worldwide has reached 537 million in 2021, of which type 2 diabetes mellitus (T2DM) accounts for the vast majority [
1,
2], and the total incidence will continue to surge with the aging of the world population. T2DM has become a major challenge for people’s health and has caused a worldwide economic burden. In the development process of T2DM, as time goes on, the clinical symptoms of “three more and one less” (food intake and water intake, fasting blood glucose, weight loss) often appear [
3]. Currently, dietary intervention, intensive exercise programs, insulin, and oral hypoglycemic agents are the most common approaches to combat T2DM, while the use of medicinal and food homologous functional foods for intervention is believed to be more beneficial in alleviating T2DM, with fewer side effects. The theory of “medicine and food homology” was formally proposed in the 1920s and 1930s [
4]. Medicinal and food homologous plants are often developed into functional foods due to being rich in bioactive compounds, which have health-promoting effects with minimal side effects. As a low glycemic index product,
Pueraria thomsonii Radix has been used as food and medicine for thousands of years in Asian countries such as China, Thailand, Vietnam, and Japan.
Pueraria thomsonii Radix is a traditional Chinese herbal medicine that can be used to treat “wasting and thirsting disorder” according to the Chinese Pharmacopoeia [
5]. Furthermore, modern studies have emphasized that the bioactive components derived from
Pueraria thomsonii Radix can help to improve fasting blood glucose in db/db mice, with limited side effects [
6,
7]. In previous studies, many monomer components of polyphenols extracted from
Pueraria thomsonii Radix, such as puerarin, daidzein, and genistein, or effective parts such as polysaccharides, have been shown to regulate animal insulin resistance to control blood glucose [
8,
9], but there is no evidence that the effect of monomer components or effective parts is better than the overall effect of
Pueraria thomsonii Radix [
7,
8,
9,
10,
11]. Therefore, a comprehensive understanding of the comprehensive mechanism for preventing the development of T2DM in
Pueraria thomsonii Radix still has limitations. We speculate that PTR, as a water extract of
Pueraria thomsonii Radix, contains both polyphenols and polysaccharides, which can better represent its overall efficacy and contribute to the development of functional foods.
Mounting evidence has demonstrated that a combination of metabolism and gut microbiota may serve as a potentially crucial factor in T2DM diagnosis, pharmaceutical discovery, as well as therapeutic response monitoring [
10,
11]. In the present study, due to deficient leptin receptor genes, db/db mice can spontaneously develop obesity and chronic hyperglycemia; thus, we used db/db mice as T2DM model mice and used db/m mice as the normal control mice, then developed PTR water extract and performed pharmacological analysis after intra-gastric administration. We compared PTR with metformin in meliorating the clinical pathological feature of db/db mice, and then explored the gut microbiota and host metabolomic reactions through UPLC-IM-Q-TOF-MS [
12,
13] and 16S rRNA gene sequencing to understand the comprehensive antidiabetic mechanism of PTR. We further propose the potential mechanism through which PTR participates in metabolic regulation through the microbiota–gut–brain axis (MGB).
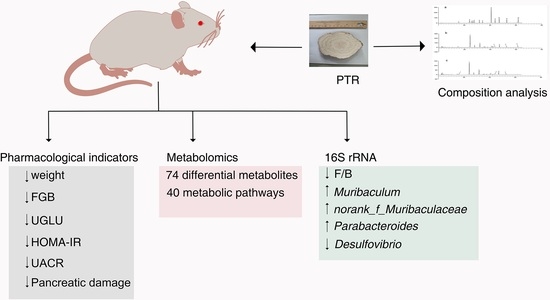
Figure 1 summarizes the schematic diagram of this study.
3. Discussion
Since ancient times, Pueraria thomsonii Radix has been known as “Asian Ginseng”, and it is a traditional Chinese herbal medicine that can be used to raise body fluids and quench thirst according to the Chinese Pharmacopoeia [
5]. Regarding physiological and biochemical indexes, the body weight and FBG of the DM group were significantly higher than that of the NC group in the first weekend of this study, indicating that db/db mice have met the conditions to serve as T2DM model mice after 3 days of adaptive feeding (
Figure 2a,b). The typical symptoms of T2DM are polydipsia, polyuria, hyperglycemia, and weight loss [
5]. Our results showed that the weight gain in the DM group is slower in the early stage and even leads to weight loss in the later stage, while the FBG increases faster, which is consistent with the symptom description of “three more and one less” in T2DM reported by multiple scholars in the prophase [
14,
15]. After the treatment with PTR and metformin, there was no significant difference in the body weight of each group of db/db mice at the weekend of every week. In addition, FBG saw a significant decrease from the third weekend of treatment. Moreover, physiological and biochemical indexes such as FINS, HOMA-IR, UGLU, and UACR in the PTR and MET groups were significantly lower than those in the DM group at the end of the ninth week after euthanasia, as well as pancreatic tissue damage, which indicated that PTR and metformin improve insulin resistance and leptin resistance in db/db mice. This is in line with previous studies showing that the thirst-quenching effects of PTR are associated with improved insulin resistance [
15]. Metformin is often used to treat T2DM patients who are unsatisfied with dietary control alone and is also commonly used in the development of new anti-diabetic drugs to evaluate the efficacy of new drugs against diabetes. In experimental diabetes models, metformin was effective in downregulating blood glucose levels and improving insulin resistance in diabetic mice and rats. In this study, our pharmacodynamic experiments showed that PTR is similar to metformin in treating T2DM by decreasing FBG, FINS, HOMA-IR, UGLU, UACR, and pancreatic tissue damage in db/db mice. This further suggests that the anti-diabetic potential of PTR is in urgent need of further clinical studies, or the use of dietary therapy to control diabetes.
Regulating metabolism is an effective measure for the treatment of diabetes. In this study, PTR reversed 74 of the 109 differential metabolites in db/db mice, mainly including purines, pyrimidines, glycerophospholipids, and steroids. Purine and pyrimidine metabolism are central to the adenosine system and play a key role in the regulation of glucose homeostasis as well as the pathophysiology of diabetes [
16,
17]. In this study, PTR affected the purine metabolic pathway by regulating the levels of Inosine, Inosinic acid, and Deoxyguanosine in db/db mice, and the pyrimidine metabolic pathway by regulating the concentrations of Orotidylic acid, dUMP, and 5-Thymidylic acid, thereby improving T2DM. Abnormal glycerophospholipid metabolism is a clinical feature of adult-onset T2DM [
18]. In this study, PTR could modulate PE (20:3(8Z,11Z,14Z)/P-18:0), PC (22:4(7Z,10Z,13Z,16Z)/P-16:0), and LysoPA (18:1(9Z)/0:0) concentrations in db/db mice. Previous studies have shown that the up-regulated release of cortisol is implicated in a hyperglycemic response and associated with diabetes-related distress in people with T2DM [
19,
20], and corticosterone may induce insulin resistance in rat hepatocytes [
21]. In this experiment, db/db mice showed a significant increase in serum cortisol and a strong positive correlation with
Desulfovibrio, a harmful bacteria in the intestinal tract whose increased abundance can cause inflammation and insulin resistance [
22], while PTR could effectively reduce the levels of serum cortisol and
Desulfovibrio in the intestinal tract, thus effectively alleviating T2DM.
There is growing evidence that dysbiosis of the intestinal flora is a typical feature of the pathogenesis of diabetes. Clinical studies have found that
Bifidobacterium, Bacillus spp.,
Clostridium pretense, and
Ackermannia spp. are negatively associated with T2DM, and that
Clostridium tumefaciens, Clostridium perfringens, and
Braunschweiger spp. are positively associated with T2DM. In animal models of experimental diabetes, the species and number of beneficial bacteria were significantly lower, while pathogenic bacteria were significantly higher [
23]. A study has demonstrated that the
Plukenetia volubilis L. leaf significantly increased
Muribaculum abundance in db/db mice, a beneficial bacterium that can improve inflammation, dyslipidemia, and glucose intolerance [
24,
25]. In this study, we found that PTR significantly upregulated the abundance of
Muribaculum,
Norank_f_Muribaculaceae, and
Parabacteroides in db/db mice, while significantly reducing the abundance of the harmful bacterium
Desulfovibrio.
Norank_f_Muribaculaceae is a beneficial dominant bacterium in the mouse intestine and is negatively correlated with the glucose levels of postprandial blood [
26].
Parabacteroides is a beneficial bacterium that significantly improves insulin resistance and has been shown in vitro to activate the intestinal gluconeogenic pathway through the conversion of succinate, thereby exerting a hypoglycaemic effect [
27]. Previous studies have reported a positive association between the
F/B ratio and the incidence of obesity and diabetes [
24,
25]. Some studies have shown that obese animals and humans exhibit higher
F/B ratios in their gut microbiota compared to individuals with normal body mass [
28]. In our study, compared with the NC group, the relative abundance of
Firmicutes in the DM group increased without a significant difference, while the relative abundance of
Bacteroidetes decreased with a significant difference. However, there was no significant difference in the decrease in the F/B ratio. However, the F/B ratio in the PTR group decreased with no significant difference compared with DM group, but the decreasing trend of the F/B ratio after PTR administration was consistent with other anti-T2DM drugs previously reported.
In addition, Spearman’s correlation analysis further revealed a positive correlation between
Desulfovibrio and Cortisol in db/db mice. Cortisol is an important product of the steroid hormone biosynthetic pathway and is considered an active indicator of the hypothalamic–pituitary–adrenal (HPA) axis. A recent study has shown that cortisol is associated with dyslipidemia and impaired glucose metabolism [
29].
Desulfovibrio has been reported to be negatively correlated with γ-aminobutyric acid (GABA) [
30], which can inhibit glucagon secretion and enhance insulin secretion [
31]. Notably, hypothalamic Cortisol concentrations are influenced by GABA, and reduced GABA levels lead to over-activation of the HPA axis, resulting in increased Cortisol levels [
32,
33]. Recent research hotspots have reported a stronger bidirectional communication between the gut and brain through the nervous, endocrine, and immune systems, i.e., MGB [
34,
35]. In this experiment, PTR reduced the abundance of
Desulfovibrio and the levels of Cortisol metabolism. Therefore, we speculate that PTR administration resulted in a decrease in
Desulfovibrio abundance, leading to an increase in GABA levels in the gut, which in turn inhibited HPA axis activation via the enteric nervous system (ENS) via the MGB pathway, contributing to a decrease in Cortisol metabolism levels (
Figure 9). This is the focus of our subsequent studies, and perhaps related studies will be reported in the near future.
Regarding the chemical composition, the content of polysaccharides and puerarin were the highest two components, and were significantly higher than the sum of other polyphenols in this study (
Table 1), which is consistent with previous research that found that both polysaccharides and polyphenols improve T2DM [
7,
8,
9,
10,
11]. In addition, our collaborative research group found that polysaccharides from Puerariae thomsonii Radix can improve fatty liver and hyperglycemia by regulating the intestinal flora and metabolites [
36,
37]. In this study, we found that PTR can reduce the
F/B ratio and regulate three beneficial bacteria and one harmful bacterium to exert hypoglycemic effects through the regulation of the nervous system, possibly mainly due to the role of its polysaccharides. However, more previous research supports that polyphenols can exert hypoglycemic effects by improving insulin resistance [
7,
8,
9,
10,
11]. Therefore, we hypothesized that PTR, as a water extract of
Pueraria thomsonii Radix, contains both polyphenols and polysaccharides, which may play a synergistic role in improving T2DM. Next, we will collaborate with another research group to investigate the differences in drug efficacy between the polysaccharide group, polyphenol group, and PTR group (containing both polysaccharides and polyphenols), in order to elucidate whether polyphenols and polysaccharides have synergistic effects and differences in improving T2DM.
4. Materials and Methods
4.1. Preparation of PTR
Pueraria thomsonii Radix, purchased from Jiangzhong Traditional Chinese Medicine Co., Ltd. (Nanchang, China), was identified as the dried root of the legume plant
Pueraria Thomsonii Benth by Professor Fei Ge and Professor Ronghua Liu of Jiangxi University of Chinese Medicine. PTR was extracted by Jiangxi Xinglin Baima Pharmaceutical Co., Ltd., Nanchang, China. A total of 200 kg of raw material was extracted 3 times continuously with 15 times the amount of water each time, filtered in a basket, combined with the filtrate, and concentrated under reduced pressure. After vacuum drying, 29.4 kg of PTR (yield: 14.70%) was obtained by crushing, which was used for subsequent animal experiments and preparation of the test solution. In our previous experiments, the UPLC-Q-TOF/MS technique was used to analyze the absorbed components of PTR in rat blood, mainly including various polyphenols (
Supplementary Table S1) [
38]. In this experiment, nine kinds of polyphenols and total polysaccharides related to T2DM were selected for qualitative analysis through previous literature investigation [
7,
8,
9,
10,
11].
4.2. Preparation of Test Solution
PTR was converted into 2 g of raw material of
Pueraria Thomsonii Radix. Afterwards, it was placed in a conical flask with a stopper, added 50% methanol (50 mL), weighed, heated in an ultrasonic bath for 60 min, cooled, weighed again, made up the lost weight with 50% methanol, shook well, and filtered through a PTFE membrane (0.22 μm) (Tianjin Zinteng Experimental Equipment Co., Ltd., Tianjin, China). The filtered solution of PTR was tested for the presence of various polyphenols (
Supplementary Table S1) [
38].
Water extraction and the alcohol precipitation method were used for the extraction and separation of polysaccharides from PTR. PTR was carefully weighed (0.0441 g), anhydrous ethanol was slowly added to the sample until no more precipitate was produced, and the samples were shaken and mixed. After 30 min of ultrasonic extraction, the samples were centrifuged for 10 min, the supernatant was taken again, and the insoluble substance was mixed twice. The insoluble substance was washed with 10 mL of 80% ethanol solution and centrifuged for 10 min. We transferred the insoluble substance to a beaker, added 50 mL of ultra-pure water to dissolve, sonicated for 30 min, and repeated twice. We cooled, filtered, transferred the filtrate in a 100 mL volumetric flask, washed the residue 2~3 times, and held the volume of ultra-pure water to obtain the solution to be tested for the determination of polysaccharides.
4.3. Preparation of Reference Solution
The nine polyphenols were purchased from Shanghai Meryer Chemical Technology Co., Ltd., Shanghai, China. We accurately weighed the above substances, added methanol to prepare a mixed reference stock solution with corresponding mass concentration, and diluted it step by step to obtain a series of mixed reference solutions with nine polyphenols in different mass concentrations.
D-anhydrous glucose standard (Shanghai Meryer Chemical Technology Co., Ltd., Shanghai, China) was taken, accurately weighed, dissolved in distilled water to volume, and prepared into a D-anhydrous glucose reference solution with a concentration of 1 mg/mL for the determination of polysaccharide content. The standard d-anhydrous glucose was weighed accurately, dissolved in distilled water to scale, and prepared into a 1 mg/mL D-glucose control solution, which was used to draw the standard curve and determine the content of polysaccharides.
4.4. Determination of PRT Active Ingredient Content
The quantitative analysis of polyphenols was performed on an Agilent 1260 Infinity II liquid chromatography system using a Cosmosil cholester (Santa Clara, CA, USA, 250 mm × 4.6 mm, 5 μm). The chromatographic column was used for HPLC analysis with a flow rate of 1 mL/min. The injection volume was 10 µL. The column temperature was 30 °C. The DAD detection wavelength was 254 nm. The mobile phase used was 0.1% formic acid in water (A) and acetonitrile (B). The gradient elution was as follows: 0–3 min, 5–11% (B); 3–15 min, 11% (B); 15–25 min, 11–15% (B); 25–35 min, 15–20% (B); 35–40 min, 20–30% (B); 40–55 min, 30–50% (B); and 55–65 min, 50–80% (B). Precision measurement: the mixed reference solution of a certain concentration prepared in
Section 4.3 was accurately injected and repeated six times. Repeatability test: a total of six injections of the test solution were prepared for the same sample. Stability test: a sample was taken and injected at 0, 1, 2, 4, 16, and 24 h. A calibration curve for linearity analyses was established by injecting each concentration of the control solution (reference solutions in
Section 4.3) under the chromatographic conditions indicated above, whereby the peak area (Y) corresponded to the ordinate and the control injection amount (X) indicated the abscissa. The peak areas of nine polyphenols were substituted into linear equations to calculate the percentage contents of each of the nine polyphenols of PTR.
The content of the polysaccharides in PTR was determined by colorimetry using a microplate detection system with SpectraMax 190 microplate (Shanghai Meigu Molecular Instrument Co., Ltd., Shanghai, China). The specific approach was as follows: firstly, precision, repeatability, and stability experiments were conducted. Subsequently, we diluted the D-glucose reference solution in
Section 4.3 to six gradients of the concentrated solution, added 200 μL of 5% phenol solution to each 200 μL, then quickly added 1 mL of concentrated sulfuric acid, let it stand for 10 min, mixed well with vortex oscillation, used 1.0 mL of ultrapure water as the blank control, and then placed the test tube in a water bath at 30 °C for 20 min. We measured the absorbance at 490 nm, using the concentration (mg/mL) as the horizontal axis and the absorbance value as the vertical axis to draw a standard curve. Under the same conditions, we accurately absorbed 200 μL of the PTR test solution prepared in
Section 4.2, used distilled water as a blank control, and measured the absorbance of the PTR test solution using the same method as the previous standard curve. Finally, we converted the total polysaccharides content using the correction coefficient of the glucose standard.
4.5. Animals and Sample Collection
The treatment of animals during the experiment was in accordance with the eighth edition of the Regulations on the Management of Laboratory Animals of China and was approved by the Ethics Committee of the Laboratory Animal Science and Technology Center of Jiangxi University of Chinese Medicine (Approval No. JZLLSC20210075). The 8-week-old male db/db mice and db/m mice (raised under the same conditions, age, and homology in an SPF environment) were obtained from Changzhou Cavins Biotech Corporation, Ltd. [Animal Certificate Number: SCXK (Su) 2016-0013]. Measuring and comparing FBG of db/dm and db/m mice, we confirmed that db/m mice could serve as T2DM model mice and subsequently grouped the animals as follows: 11 db/m mice were used as Normal Control (NC) group and 39 db/db mice were modeled for T2DM and randomly divided into three groups, with 13 mice in each: Diabetes Control (DM) group, PTR group, and Metformin (MET) group. Then, they were raised in the protective environment of the Animal Science and Technology Experimental Center of Jiangxi University of Chinese Medicine (humidity: 50 ± 5%, temperature: 22 ± 2 °C, and light-dark cycle for 12 h).
After three days of adaptive feeding, the body weight and FBG of each group were measured as their first weekend data, then each group was given corresponding drugs by intragastric administration according to their weight, once a day for 8 weeks, and their weight and FBG were measured at the end of every week. Namely, the mice in the NC group and DM group were intragastrically administered with distilled water, the mice in the PTR group were intragastrically administered with PTR 0.89 g/kg/d (according to the formula of human and mouse body surface area, the most commonly used dosage for human administration and the yield of extract powder prepared from raw drug), and the mice in the MET group were intragastrically administered with the original drug of metformin 0.364 g/kg/d (Sino American Shanghai Squibb Pharmaceutical Co., Ltd., China).
On the night before the ninth weekend, we started fasting and water prohibition at 18:00. On the morning of the ninth weekend, about 50 μL of blood was collected from the orbital venous plexus of the mice and put into common EP tubes for the determination of FINS. One hour after the last administration, mice were anesthetized and whole blood was collected from abdominal aorta into common EP tubes and centrifuged at 8000 r/min for 15 min in a cryogenic centrifuge at 4 °C. After centrifugation, the supernatant was collected and stored at −80 °C for serum metabolomics study. After euthanizing, samples of the pancreatic and intestinal contents were carefully collected and then flash-frozen in liquid nitrogen and stored at −80 °C for subsequent experimental analysis.
4.6. Analysis of Physiological and Biochemical Indexes
The weekly body weight of mice was measured using an electronic analytical balance (Beijing Sartorius Scientific Instruments Co., Ltd., Beijing, China). The levels of FBG were measured weekly using a blood glucose meter and a blood glucose strip (Roche Blood Glucose Health Care). FINS were measured using the Mouse Insulin (INS) Elisa kit (Nanjing Jiancheng Bioengineering Institute, China). HOMA-IR was calculated using the following formula: FBG × FINS/22.5. UGLU was measured using an automatic biochemical analyzer (Tokyo, Japan, Hitachi7180). UACR was measured by an automatic biochemical analyzer from Catalyst (Wellington, New Zealand).
4.7. Observation of Pancreatic Hematoxylin-Eosin (HE) Staining
The pancreatic samples were fixed in a 10% formalin solution. A paraffin-embedding machine and microtome (Leica, Wetzlar, Germany) were used to embed and slice the tissue to a thickness of 4 μm. The sections were routinely stained with HE, sealed with neutral gum, and mounted on a glass slide and examined under a light microscope (Mshot, Beijing, China) with 400× total magnification. Based on the pathological changes in the understudied tissues, the histological sections were rated approximately on a scale from 0 to 4, with no change (0), minimal changes (+1), mild changes (+2), moderate changes (+3), and severe changes (+4).
4.8. Untargeted Metabolomics Analysis
We thawed the frozen serum samples naturally, took 50 μL of serum, added 200 μL of the methanol working solution (containing 10.06 µg/mL 2-chloro-L-phenylalanine), mixed them using the MTV-100 vortex mixer (Hangzhou Aosheng Group Co., Ltd., Hangzhou, China) for 30 s, and after standing for 10 min, centrifuged at 12,000 r/min for 15 min at 4 °C. The supernatant was taken for untargeted metabolomic analysis in UPLC-IM-Q-TOF-MS (UPLC I-CLASS liquid chromatography system and SYNAPT G2-Si mass spectrometer, Waters, MA, USA). In addition, quality control (QC) samples were prepared by taking an equal amount from each frozen serum sample, vortexed and mixed according to the above method.
Liquid chromatographic separation of the samples was achieved using an ACQUITY UPLC BEN C18 column (2.1ITY UPLC BEm, Waters Corporation). The injection volume was 2 µL, the column temperature was 40 °C, the flow rate was 0.35 mL/min, the eluent A was water containing 0.1% formic acid, and the eluent B was acetonitrile. Solvent gradient setting in positive ion mode: 0–0.2 min, 5% B; 0.2–2 min, 5–20% B; 2–7 min, 20–50% B; 7–17 min, 50–65% B; 17–22 min, 65–80% B; 22–23 min, 80–95% B; 23–26 min, 95–5% B; negative ion mode: 0–0.2 min, 5% B; 0.2–3 min, 5–20% B; 3–5 min, 20–45% B; 5–7 min, 45–55% B; 7–13 min, 55–65% B; 13–16 min, 65% B; 16–21 min, 65–80% B; 21–23 min, 80–95% B; 23–26 min, 95–5% B.
An ESI ion source was used to carry out data acquisition in positive and negative ion mode in HDMSE mode. The mass range (m/z) was 50–1200 Da, ion source temperature was 120 °C, curtain rate cone gas flow rate was 50 L/h, desolvation gas flow rate was 800 L/h, desolvation temperature was 400 °C, cone voltage was 40 V, collision energy was 20–40 V, scan time and inter-scan delay were 0.3 s and 0.015 s, capillary ESI+ was 3 kV, and ESI- was 2.3 kV. In order to ensure the accuracy and reproducibility of the experimental data, the standard product sodium formate was used to establish the mass axis standard curve, and at the same time, leucine enkephalin was used for real-time mass correction, and polyalanine (purchased from Sigma p/n P9003) was used to perform CCS Correction.
In this experiment, in order to ensure the stability of the analysis system as a whole, method validation was performed using quality control (QC) samples. Before collecting each group of samples, QC samples were advanced five times to balance the instrument. After stabilization, one QC sample was injected for every five experimental samples to monitor the operation of the system in real-time. The real-time monitoring system should be run to analyze the data in real-time and evaluate the data quality. Finally, the peak intensities of 10 typical mass spectrometry peaks were analyzed, the value of relative standard deviation (RSD) was used to confirm that the instrument injection was stable, and the method was reproducible.
The mass spectrometry raw data obtained by UPLC-IM-QTOF-MS were imported into Progenesis QI V2.0 software (QI, Waters, USA) for normalization processing, and then the data matrix was exported and saved. After grouping the data, we exported the data to the EZinfo3.0 (Waters, USA) software to establish labels (VIP > 1 and
p < 0.05) to screen differential metabolites. The SIMCA14.1 (Swedish Umetrics Company) software was used to perform principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) on the data obtained in the previous step. According to the data of the mass-to-charge ratio, retention time, CCS value, and secondary mass spectrometry fragments of the differential metabolites, they were matched and identified in the HMDB database, and then were analyzed by Pathway Analysis in the Metabo Analyst 5.0 (
https://www.metaboanalyst.ca) (accessed on 18 October 2022) online database. The identified differential metabolites were enriched for metabolic pathways, and the differential metabolites were finally determined to obtain metabolic pathways and conduct pathway analysis.
4.9. Gut Microbiota Analysis
Gut microbiota analysis was performed by Shanghai Magi Biomedical Technology Co., LTD. (Shanghai, China). We thawed the frozen samples of the intestinal contents naturally and DNA was extracted from differential samples of intestinal contents using E.Z.N.A.
® Soil DNA Kit (Omega Biotek, Norcross, GA, USA). The quality of DNA extraction was examined using 1% agarose gel electrophoresis, and the concentration and purity of the DNA were determined using a NanoDrop2000 ultramicro spectrophotometer (Thermo Scientific, Wilmington, USA). The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) by an ABI GeneAmp
® 9700 PCR thermocycler (ABI, California, USA). There were three replicates for each sample. The PCR products of each sample were mixed in triplicate and then the PCR products were recovered on 2% agarose gel. AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) was used to purify the recovered product and detect it by 2% agarose gel electrophoresis. Quantus™ Fluorometer (Promega, Wisconsin, USA) was used to detect and quantify the recovered products. The NEXTflexTM Rapid DNA-Seq Kit (Bioo Scientific, Texas, USA) was used for library construction. Sequencing was performed using Illumina’s Miseq PE300 platform. The fastp software (
https://github.com/OpenGene/fastp, version 0.20.0) (accessed on 5 November 2022) was used for quality control of original sequencing sequence. FLASH software (
http://www.cbcb.umd.edu/software/flash, version 1.2.7) (accessed on 5 November 2022) was used to pick up the fight. UPARSE software (
http://drive5.com/uparse/,Version 7.1) (accessed on 5 November 2022) was used to cluster OTU sequences according to a 97% similarity. RDP classifier (
http://rdp.cme.msu.edu/, version 2.2) (accessed on 5 November 2022) was used to annotate each sequence for species classification. The comparison threshold was set to 70% for Silva 16S rRNA database (V138).
Statistical analyses. These values were defined as mean ± standard deviation (SD). The difference between the two groups of metabolites was calculated using student’s t-test by GraphPad prism 9 (GraphPad Software Inc., La Jolla, CA, USA). The difference between the two groups of gut microbiota was calculated using the Wilcox rank-sum test. Compared with the NC group or DM group, the significance of the difference was described as * p < 0.05, ** p < 0.01, and *** p < 0.001.