In Situ Formation of a Relatively Transparent Ion-Associate Liquid Phase from an Aqueous Phase and Its Application to Microextraction/High-Performance Liquid Chromatography–Fluorescence Detection of Bisphenol A in Water
Abstract
:1. Introduction
2. Results
2.1. Organic Ions Constituting the Ion-Associate Liquid Phase (IALP)
2.1.1. HPLC-FLD
2.1.2. HPLC-ECD
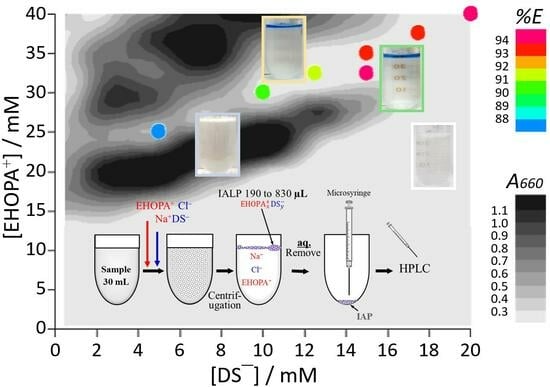
2.2. Regions of Ion-Associate (IA) and Liquid Phase (LP) Formation
2.3. Effect of Organic Ions on Ion-Associate Liquid Phase (IALP) Volume, Percent Extraction (%E), and Distribution Constant (Kd)
2.4. Analytical Figure of Merit
2.5. Application to River Water Samples
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Apparatus
4.3. Ion-Associate (IA) and Liquid Phase (IALP) Formation
4.4. Ion-Associate Liquid Phase (IALP) Microextraction
4.5. Measurement of IALP Volume
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sajid, M. Advances in on-site analytical sample preparation for analysis of environmental waters: A review. TrEAC Trends Env. Anal. Chem. 2022, 36, e00175. [Google Scholar] [CrossRef]
- Tazoe, H. Water quality monitoring. Anal. Sci. 2023, 39, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lu, W.H.; Liu, H.T.; Wu, X.G.; Li, J.H.; Chen, L.X. Dispersive liquid-liquid microextraction for four phenolic environmental estrogens in water samples followed by determination using capillary electrophoresis. Electrophoresis 2016, 37, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Kuramitz, H.; Hata, N.; Taguchi, S.; Murai, K.; Okauchi, K. Visual colorimetry for determination of trace arsenic in groundwater based on improved molybdenum blue spectrophotometry. Anal. Methods 2015, 7, 2794–2799. [Google Scholar] [CrossRef]
- Ministry of the Environment, Japan. Environmental Quality Standards for Water Pollution. Available online: https://www.env.go.jp/en/water/wq/wp.pdf (accessed on 23 June 2023).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st and 2nd addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. [Google Scholar]
- Ministry of the Environment, Japan. National Effluent Standards. Available online: https://www.env.go.jp/en/water/wq/nes.html (accessed on 23 June 2023).
- Yang, X.; Diao, C.P.; Sun, A.L.; Liu, R.M. Rapid pretreatment and determination of bisphenol A in water samples based on vortex-assisted liquid–liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J. Sep. Sci. 2014, 37, 2745–2750. [Google Scholar] [CrossRef]
- Li, T.; Song, Y.; Dong, Z.; Shi, Y.; Fan, J. Hydrophobic deep eutectic solvents as extractants for the determination of bisphenols from food-contacted plastics by high performance liquid chromatography with fluorescence detection. J. Chromatogr. A 2020, 1621, 461087. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, M.; Qin, Y.; Zhou, Y. Hydrophobic deep eutectic solvent based dispersive liquid–liquid microextraction for the preconcentration and HPLC analysis of five rice paddy herbicides in water samples. Microchem. J. 2022, 181, 107790. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Elmansi, H.; Belal, F.; Magdy, G. Recent advances in dispersion strategies for dispersive liquid–liquid microextraction from green chemistry perspectives. Microchem. J. 2023, 191, 108807. [Google Scholar] [CrossRef]
- Manaka, A.; Sawada, M.; Tafu, M. Simple and High-sensitivity Analysis of Small Microplastics with Phase Separation by Using Water/isopropanol/chloroform. Chem. Lett. 2023, 52, 420–421. [Google Scholar] [CrossRef]
- Tani, H.; Kamidate, T.; Watanabe, H. Micelle-mediated extraction. J. Chromatogr. A 1997, 780, 229–241. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Ning, J.; Yang, Y. Cloud point extraction for the determination of bisphenol A, bisphenol AF and tetrabromobisphenol A in river water samples by high-performance liquid chromatography. Anal. Methods 2014, 6, 3285–3290. [Google Scholar] [CrossRef]
- Yıldırım, E.; Gürkan, R.; Altunay, N. A new ultrasonic thermostatic-assisted cloud point extraction/spectrophotometric method for the preconcentration and determination of bisphenol A in food, milk, and water samples in contact with plastic products. Food Anal. Methods 2017, 10, 1765–1776. [Google Scholar] [CrossRef]
- Haq, H.U.; Elik, A.; Durukan, H.; Sarac, H.; Ahmet Demirbas, A.; Boczkaj, G.; Gürsoy, N.; Altunay, N. Application of chemometric modeling for ionic liquid-based ultrasonic-assisted dispersive liquid-liquid microextraction: Analysis of fosetyl-aluminum in fruit and vegetable samples. J. Food Compos. Anal. 2023, 124, 105725. [Google Scholar] [CrossRef]
- Li, K.; Jin, Y.; Jung, D.; Park, K.; Kim, H.; Lee, J. In situ formation of thymol-based hydrophobic deep eutectic solvents: Application to antibiotics analysis in surface water based on liquid-liquid microextraction followed by liquid chromatography. J. Chromatogr. A 2020, 1614, 460730. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Jiang, Y.; Qin, Z.; Ma, P.; Sun, Y.; Wang, X.; Song, D.; Fei, Q. Application of an in-situ formulated magnetic deep eutectic solvent for the determination of triazine herbicides in rice. Talanta 2021, 222, 121527. [Google Scholar] [CrossRef]
- Hata, N.; Kasahara, I.; Taguchi, S. Micro-phase sorbent extraction for trace analysis via in situ sorbent formation: Application to the preconcentration and the spectrophotometric determination of trace ammonia. Anal. Sci. 2002, 18, 697–699. [Google Scholar] [CrossRef]
- Hata, N.; Yuwatini, E.; Ando, K.; Yamada, M.; Kasahara, I.; Taguchi, S. Micro-organic ion-associate phase extraction via in situ fresh phase formation for the preconcentration and determination of di(2-ethylhexyl)phthalate in river water by HPLC. Anal. Sci. 2004, 20, 149–152. [Google Scholar] [CrossRef]
- Hata, N.; Hieda, S.; Yamada, M.; Yasui, R.; Kuramitz, H.; Taguchi, S. Formation of a liquid organic ion associate in aqueous solution and its application to the GF-AAS determination of trace cadmium in environmental water as a complex with 2-(5-bromo-2-pyridylazo)5-(N-propyl-N-sulfopropylamino)phenol. Anal. Sci. 2008, 24, 925–928. [Google Scholar] [CrossRef]
- Mizuna, K.; Murashima, R.; Okazaki, T.; Sazawa, K.; Kuramitz, H.; Taguchi, S.; Nakayama, K.; Yamamoto, T.; Takamura, Y.; Hata, N. Organic Ion-associate Phase Extraction/Back-microextraction for the Preconcentration and Determination of Lithium Using 2,2,6,6-Tetramethyl-3,5-heptanedione by Liquid Electrode Plasma Atomic Emission Spectrometry and GF-AAS in Environmental Water. Anal. Sci. 2020, 36, 595–600. [Google Scholar] [CrossRef]
- Kosugi, M.; Mizuna, K.; Sazawa, K.; Okazaki, T.; Kuramitz, H.; Taguchi, S.; Hata, N. Organic Ion-Associate Phase Microextraction/Back-Microextraction for Preconcentration: Determination of Nickel in Environmental Water Using 2-Thenoyltrifluoroacetone via GF-AAS. Appl. Chem. 2021, 1, 130–141. [Google Scholar] [CrossRef]
- Hata, N.; Igarashi, A.; Matsushita, M.; Kohama, N.; Komiyama, T.; Sazawa, K.; Kuramitz, H.; Taguchi, S. Evaluation of an Ion-Associate Phase Formed In Situ from the Aqueous Phase by Adding Benzethonium Chloride and Sodium Ethylbenzenesulfonate for Microextraction. Appl. Chem. 2023, 3, 32–44. [Google Scholar] [CrossRef]
- Ministry of Economy, Trade and Industry (METI), Japan. Yearbook of Current Production Statistics 2019: Chemical Industry. Available online: https://www.meti.go.jp/statistics/tyo/seidou/result/gaiyo/resourceData/02_kagaku/nenpo/h2dbb2019k.pdf (accessed on 31 August 2023).
- European Chemicals Agency (ECHA). All News: Four New Substances of Very High Concern Added to the Candidate List. Available online: https://echa.europa.eu/-/four-new-substances-of-very-high-concern-added-to-the-candidate-list (accessed on 31 August 2023).
- Ministry of the Environment, Japan. Environmental Risk Assessment of Chemical Substances, 15. Bisphenol A 2004, 20 Pages. Available online: https://www.env.go.jp/chemi/report/h16-01/pdf/chap01/02_2_15.pdf (accessed on 31 August 2023).
- Ministry of Health, Labour and Welfare, Japan. Drinking Water Quality Standards, Water Quality Control Target Setting Items (Pollutants). Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/topics/bukyoku/kenkou/suido/kijun/kijunchi.html#01 (accessed on 8 September 2023).
- Water Quality Management Division, Water Quality Protection Bureau, Environment Agency, Tentative Manual for Investigation of Exogenous Endocrine Disrupting Chemicals (Water Quality, Sediment, Aquatic Life), 1998. Available online: https://www.env.go.jp/chemi/end/sympo/manual199810/water.html (accessed on 12 September 2023).
- Hahladakis, J.N.; Iacovidou, E.; Gerassimidou, S. An overview of the occurrence, fate, and human risks of the bisphenol-A present in plastic materials, components, and products. Integr. Environ. Assess Manag. 2023, 19, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Czarny-Krzymińska, K.; Krawczyk, B.; Szczukocki, D. Bisphenol A and its substitutes in the aquatic environment: Occurrence and toxicity assessment. Chemosphere 2023, 315, 137763. [Google Scholar] [CrossRef]
- Yao, Y.; Shao, Y.; Zhan, M.; Zou, X.; Qu, W.; Zhou, Y. Rapid and sensitive determination of nine bisphenol analogues, three amphenicol antibiotics, and six phthalate metabolites in human urine samples using UHPLC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare, Japan. Safety Data Sheet. Dichloromethane. Available online: https://anzeninfo.mhlw.go.jp/anzen/gmsds/75-09-2.html (accessed on 12 September 2023).
- Kasahara, I.; Ohgaki, Y.; Matsui, K.; Kano, K.; Taguchi, S.; Goto, K. Assignment of Individual Ion-Pair Extraction Constants Based on the KPh4As+ = KPb4B-; Kbenzene = 1 Assumption. Nippon Kagaku Kaishi 1986, 1986, 894–900. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA, Washington, DC, USA). KOWWIN 1.68 in “Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11.” 2012. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 8 September 2023).
- Zhavoronok, M.F.; Vakh, C.; Bulatov, A. Automated primary amine-based supramolecular solvent microextraction with monoterpenoid as coacervation agent before high-performance liquid chromatography. J. Food Compos. Anal. 2023, 116, 10585. [Google Scholar] [CrossRef]
- Hata, N.; Teraguchi, K.; Yamaguchi, M.; Kasahara, I.; Taguchi, S.; Goto, K. Spectrophotometric Determination of Ammonia-Nitrogen After Preconcentration as Indothymol on a Glass-Fiber Filter in the Presence of a Cationic Surfactant. Mikrochim. Acta 1992, 106, 101–108. [Google Scholar] [CrossRef]
- Gabal, R.A.; Osama, S.; Hanafy, N.; Oraby, A. Micellization thermodynamics as a function of the temperature of a cationic zwitterionic dodecyl phosphocholine and anionic sodium dodecyl sulfate mixed micelles with fluorometry. Appl. Phys. A 2023, 129, 201. [Google Scholar] [CrossRef]







| Organic Substances | log Kanion [34] | log Kowwin [35] | Molecular Weight | |
|---|---|---|---|---|
| Name (Abbreviation) | Formula | |||
| Organic anions | as sodium salts | |||
| Bis(2-ethylhexyl) Sulfosuccinate (SS−) | (C8H17COO)2C2H3SO3− | 2.2 | 3.95 | 444.56 |
| Dodecylbenzenesulfonate (DBS−) | C12H25C6H4SO3− | 1.5 | 3.00 | 348.48 |
| Tetradecylsulfonate (C14S−) | C14H29SO3− | 0.9 | 1.86 | 300.44 |
| Dodecylsulfate (DS−) | C12H25SO4− | 1.5 | 1.69 | 288.38 |
| Substances for organic cations and analyte | ||||
| Tris[2-(2-methoxyethoxy)ethyl]amine | (CH3OC2H4OC2H4)3N | -- | 323.43 | |
| 3-Butoxypropylamine | C4H9OC3H6NH2 | -- | 1.05 | 131.22 |
| Ethylhexyloxypropylamine (EHOPA+) | C11H25NO | -- | 2.94 | 187.33 |
| N-Methyldidodecylamine | (C12H25) 2NCH3 | -- | 10.84 | 367.7 |
| BPA | C15H16O2 | -- | 3.64 | 228.29 |
| Detection | [EHOPA+]/mM | [DS−] /mM | LOD | %E | log Kd | Enrichment Factor | |
|---|---|---|---|---|---|---|---|
| /µg L−1 | nM | ||||||
| Fluorescence | 27.5 | 10 | 0.049 | 0.21 | 89 | 2.9 | 290 |
| 25 | 5 | 0.009 | 0.04 | 86 | 3.1 | 310 | |
| Electrochemical | 31 | 12 | 0.2 | 1 | 93 | 3.0 | 150 |
| Sample Preparation | Analysis Method | Sample or Matrix | Extractant | LR/µg L−1 | LOD/µg L−1 | Enrichment Factor | Reference |
|---|---|---|---|---|---|---|---|
| VALLME | HPLC-FLD | natural water | 2-ethylhexanol | 0.1–100 | 0.02 | -- | [8] |
| CPE | HPLC-UV | river water | AEO9, octanol | 0.05–20 | 0.27 | -- | [14] |
| DLLME | CE | tap water, lake water, seawater | chlorobenzene | 4–300 | 0.6 | 241 | [3] |
| UTA-CPE | UV–vis spectrophotometry at 643 nm | drinking water | Brij 35 | 1.2–160 | 0.35 | 180 | [15] |
| SPE | UHPLC-MS/MS | urine | mixed-mode anion-exchange SPE | 0.5–50 | 0.13 | -- | [32] |
| VALLME | HPLC-FLD | plastic materials | hydrophobic des (decanoic acid, trioctylmethyl ammonium chloride) | 0.3–700 | 0.06 | 110.3 | [9] |
| SUPRAS-ME | Automated HPLC-FLD | beverages | supramolecular solvent (1-hexylamine, menthol) | 2–5000 | 0.7 | 10.4 | [36] |
| IALPME | HPLC-FLD | water | ethylhexyloxypropylamine, dodecylsulfate | 0.5–5 | 0.009 | 310 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata, N.; Takahashi, S.; Osada, S.; Katagiri, S.; Naruse, M.; Igarashi, A.; Sazawa, K.; Taguchi, S.; Kuramitz, H. In Situ Formation of a Relatively Transparent Ion-Associate Liquid Phase from an Aqueous Phase and Its Application to Microextraction/High-Performance Liquid Chromatography–Fluorescence Detection of Bisphenol A in Water. Molecules 2023, 28, 7525. https://doi.org/10.3390/molecules28227525
Hata N, Takahashi S, Osada S, Katagiri S, Naruse M, Igarashi A, Sazawa K, Taguchi S, Kuramitz H. In Situ Formation of a Relatively Transparent Ion-Associate Liquid Phase from an Aqueous Phase and Its Application to Microextraction/High-Performance Liquid Chromatography–Fluorescence Detection of Bisphenol A in Water. Molecules. 2023; 28(22):7525. https://doi.org/10.3390/molecules28227525
Chicago/Turabian StyleHata, Noriko, Seira Takahashi, Sachiko Osada, Sakura Katagiri, Mayumi Naruse, Akane Igarashi, Kazuto Sazawa, Shigeru Taguchi, and Hideki Kuramitz. 2023. "In Situ Formation of a Relatively Transparent Ion-Associate Liquid Phase from an Aqueous Phase and Its Application to Microextraction/High-Performance Liquid Chromatography–Fluorescence Detection of Bisphenol A in Water" Molecules 28, no. 22: 7525. https://doi.org/10.3390/molecules28227525
APA StyleHata, N., Takahashi, S., Osada, S., Katagiri, S., Naruse, M., Igarashi, A., Sazawa, K., Taguchi, S., & Kuramitz, H. (2023). In Situ Formation of a Relatively Transparent Ion-Associate Liquid Phase from an Aqueous Phase and Its Application to Microextraction/High-Performance Liquid Chromatography–Fluorescence Detection of Bisphenol A in Water. Molecules, 28(22), 7525. https://doi.org/10.3390/molecules28227525