Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies
Abstract
:1. Methylglyoxal in (Patho)physiology
1.1. Endogenous Sources of MGO
1.2. Exogenous Sources of MGO
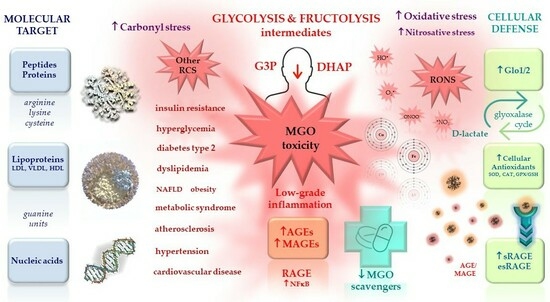
1.3. MGO Modification of Macromolecules
1.3.1. MGO-Derived AGEs (MAGEs)
1.3.2. MGO-Derived DNA Modifications
1.4. MGO Scavenging System
2. MGO and MAGEs in Metabolic Syndrome and Diabetes
2.1. Metabolic Syndrome
2.2. MGO and MAGEs in Metabolic Syndrome and Diabetes in Animal Models and Cell Cultures
MGO/MAGEs in Insulin Resistance Development
2.3. MGO, Its Metabolic Products, and MAGEs in Patients with Metabolic Syndrome and Diabetes
3. MGO and MAGEs in Cardiovascular Disorders
3.1. Pathological Routes Linking Metabolic Syndrome and Diabetes with Cardiovascular Complications
3.2. MGO/MAGEs Contribution to Blood Vessels Wall Impairment, Hypertension, Dyslipidemia and Atherosclerosis
3.2.1. Blood Vessels Focusing on Endothelium—Impairment of Angiogenesis
3.2.2. Cardiovascular System in Animal Models
3.2.3. Cardiovascular Disorders in Patients
3.2.4. Atherosclerosis
3.2.5. Endoplasmic Reticulum Stress (ER Stress) Followed by Unfolded Protein Response (UPR) in Blood Vessels
3.2.6. Hypertensive and Procoagulatory Properties of MGO/MAGE
3.2.7. Dyslipidemia
4. Potential Glycation Inhibitors and MGO Scavengers—Therapeutic Strategies
4.1. Overview of the Potential Glycation Inhibitors and MGO Scavengers
4.1.1. Oral Antihyperglycemic Agents
4.1.2. Angiotensin II Receptor Antagonists, and Angiotensin-Converting Enzyme Inhibitors
4.1.3. Calcium Channel Blockers
4.1.4. Hydrazinophthalazine Derivatives
4.1.5. Lipid Modifying Agents (Statins)
4.1.6. Peripheral Vasodilators and Vasoprotectives
4.1.7. Anti-Inflammatory, Analgesic, and Antipyretic Agents
4.1.8. Selected B Vitamins
5. Conclusions and Remarks for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| AG | aminoguanidine |
| AGEs | advanced glycation end products |
| AKRs | Aldoketo reductases |
| Akt | PKB = protein kinase B (serine/threonine kinase) |
| ALDHs | Aldehyde dehydrogenases |
| AMPK | AMP-activated kinase |
| Ang II (Ang-2) | angiotensin II |
| AP | argpyrimidine |
| apoA1 | apolipoprotein A1 |
| apoB100 | apolipoprotein B100 |
| ApoE KO | apolipoprotein E knockout |
| ATF6 | activating transcription factor 6 |
| AT2R | angiotensin II receptor type 2 |
| BBGC | bromobenzyl-glutathione cyclopentyl diester (glyoxalase-1 inhibitor) |
| BMCs | bone marrow cells |
| CAD | coronary artery disease |
| CAT | catalase |
| CEA | N7-carboxyethyl arginine |
| C/EBP | transcription factor C/EBP |
| CEdG | N2-carboxyethyl-20–deoxyguanosine |
| CEL | Nε-(1-carboxyethyl)lysine = N6-(1-carboxyethyl)lysine |
| C. elegans | Caenorhabditis elegans |
| CETP | cholesteryl ester transfer protein |
| CKD | chronic kidney disease |
| CML | Nε-(1-carboxymethyl)lysine = N6-(1-carboxymethyl)lysine |
| CTGF | connective tissue growth factor |
| CHD | coronary heart diseases |
| CVD | cardiovascular diseases |
| DAG | diacylglycerol |
| DJ-1 (PARK7) | Parkinson’s disease protein 7 |
| DKO | double knockout |
| 3-DG | 3-deoxyglucosone |
| 3DG-H | 3-DG-derived hydroimidazolones |
| EA.hy926 | hybrid human umbilical vein endothelial cell line |
| ECM | extracellular matrix |
| eNOS | endothelial nitric oxide synthase |
| EPCs | endothelial progenitor cells |
| ER | endoplasmic reticulum |
| esRAGE | endogenous secretory RAGE proteolytically exfoliated by metalloproteinases |
| FFAs | free fatty acids |
| FL | Nε-fructosyl-lysine |
| FPG | fasting plasma glucose |
| Fru | fructose |
| F4/80 | EGF-like module-containing mucin-like hormone receptor-like 1 |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| Glc | glucose |
| GlcNAc | N-acetylglucosamine |
| Glo1 | Glyoxalase 1 |
| Glo1 KO | Glo1 knockout |
| Glo2 | Glyoxalase 2 |
| GLUT | glucose transporter |
| GO | glyoxal |
| GPX | glutathione peroxidase |
| GSH | reduced glutathione |
| GSK-3 | Glycogen synthase kinase-3 |
| GSSG | oxidized glutathione |
| HAECs | human aortic endothelial cells |
| HbA1c | hemoglobin A1c |
| HEK293 | human embryonic kidney cells |
| HIF | hypoxia-inducible factor |
| HoxA5 | homeobox A5 transcription factor |
| HO-1 | heme oxygenase 1 |
| HSPG | heparan sulfate proteoglycan |
| HUVECs | human umbilical cord vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN-γ | interferon gamma |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-1β | interleukin-1 β |
| IR | insulin receptor |
| IRE1 | inositol-requiring enzyme-1 |
| IRS-1 | insulin receptor substrate 1 |
| KATP channel | ATP-sensitive potassium channel |
| KRAS | GTPase Kirsten Rat Sarcoma Viral Oncogene Homolog |
| LCAT | lecithin-cholesterol acyltransferase |
| Mac-1 | macrophage-1 antigen |
| Mac-2 | macrophage-2 antigen |
| MAECs | mouse aortic endothelial cells |
| MafA | musculoaponeurotic fibrosarcoma oncogene family A |
| MAGEs | MGO-derived AGEs |
| MCP-1 | monocyte chemoattractant peptide-1 |
| MDA | malondialdehyde |
| MG-dG | 3-(20–deoxyribosyl)-6,7-dihydro-6,7-dihydroxy-6/7-methylimidazo-[2,3-b]purin-9(8)one |
| MG-H1-3 | MGO-derived hydroimidazolones 1-3 |
| MG-H1 | Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine |
| MG-H2 | 2-amino-5-(2-amino-5-hydro-5-methyl-4-imidazolon-1-yl)-pentanoic acid |
| MG-H3 | 2-amino-5-(2-amino-4-hydro-4-methyl-5-imidazolon-1-yl)-pentanoic acid |
| MGO | methylglyoxal |
| MMP-9 | matrix metalloproteinase 9 |
| MnSOD | manganese superoxide dismutase |
| MODIC | 2-ammonio-6-((2-[(4-ammonio-5-oxido-5-oxopentyl)amino]-4-methyl-4,5-dihydro-1H-imidazol-5-ylidene)amino)hexanoate |
| MOLD | 1,3-di(Nε-lysino)-4-methyl-imidazolium |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NAC | N-acetyl cysteine |
| NFATc | Nuclear factor of activated T-cells, cytoplasmic |
| NO | nitric oxide |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor erythroid 2 related factor 2 |
| OGTT | oral glucose tolerance test |
| p38 MAPK | p38 mitogen-activated protein kinase |
| PAI-1 | plasminogen activator inhibitor 1 |
| PARP | poly(ADP-ribose) polymerase |
| Pdx1 | gene coding for pancreatic duodenal homeobox-1 |
| PDX-1 | homeodomain (HD)-containing transcription factor (syn: IPF-1 (insulin promoter factor 1) |
| PERK | double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase |
| PGC1α | transcriptional coactivator PGC1-α |
| PI3K | phosphatidylinositol (PI) 3-kinase |
| PKB/Akt | protein kinase B (serine/threonine kinase) |
| PKC | protein kinase C |
| p-JNK | phosphorylated c-Jun NH2—terminal kinase |
| p-p38 | phosphorylated p38 kinase |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| PHLPP2 | PH domain leucine-rich repeat protein phosphatase 2 |
| PON1 | paraoxonase 1 |
| PPAR | peroxisome proliferation-activated receptor |
| RAGE | AGEs receptor |
| RAAS | renin-angiotensin-aldosterone system |
| RCS | Reactive carbonyl species |
| RONS | Reactive oxygen and nitrogen species |
| sdLDL | small dense low density lipoproteins |
| SD rats | Sprague Dawley rats |
| SHR | Spontaneously hypertensive rats |
| SNP | sodium nitroprusside |
| SSAO | semicarbazide-sensitive amine oxidase |
| STZ | streptozotocin |
| sICAM-1 | soluble intercellular adhesion molecule 1 |
| SOD-(1–3) | superoxide dismutase (1–3) |
| sPLA2 | secreted phospholipase A 2 |
| sRAGE | soluble RAGE produced by alternative splicing |
| sVCAM-1 | soluble vascular cell adhesion molecule 1 |
| TAG | triacylglycerol |
| TAK1 | transforming growth factor-β-activated kinase 1 |
| T1DM | type 1 diabetes |
| T2DM | type 2 diabetes |
| TGF-β | transforming growth factor β |
| THP | tetrahydropyrimidine |
| TNF-α | tumor necrosis factor α |
| UCP-2 | uncoupling protein 2 |
| UDPGlcNAc | uridine diphosphate N-acetylglucosamine |
| UPR | unfolded protein response |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
| VSMCs | vascular smooth muscle cells |
| 8-OHdG | 8-hydroxy-2-deoxyguanosine |
| WKY | Wistar Kyoto rats |
References
- Nigro, C.; Leone, A.; Fiory, F.; Prevenzano, I.; Nicolò, A.; Mirra, P.; Beguinot, F.; Miele, C. Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging. Cells 2019, 8, 749. [Google Scholar] [CrossRef]
- Stratmann, B. Dicarbonyl Stress in Diabetic Vascular Disease. Int. J. Mol. Sci. 2022, 23, 6186. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Carbonyl Stress in Red Blood Cells and Hemoglobin. Antioxidants 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.R. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response 2009, 7, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Zemva, J.; Fink, C.A.; Fleming, T.H.; Schmidt, L.; Loft, A.; Herzig, S.; Knieß, R.A.; Mayer, M.; Bukau, B.; Nawroth, P.P.; et al. Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox Biol. 2017, 13, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M.; Priebe, S.; Grigolon, G.; Rozanov, L.; Groth, M.; Laube, B.; Guthke, R.; Platzer, M.; Zarse, K.; Ristow, M. Impairing L-Threonine Catabolism Promotes Healthspan through Methylglyoxal-Mediated Proteohormesis. Cell Metab. 2018, 27, 914–925.e5. [Google Scholar] [CrossRef]
- Masania, J.; Malczewska-Malec, M.; Razny, U.; Goralska, J.; Zdzienicka, A.; Kiec-Wilk, B.; Gruca, A.; Stancel-Mozwillo, J.; Dembinska-Kiec, A.; Rabbani, N.; et al. Dicarbonyl stress in clinical obesity. Glycoconj. J. 2016, 33, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Fleming, T.; Stoyanov, S.; Leffler, A.; Babes, A.; Neacsu, C.; Sauerj, S.K.; Eberhardt, M.; Schnölzer, M.; Lasitschka, F.; et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 2012, 18, 926–933. [Google Scholar] [CrossRef]
- McLellan, A.C.; Thornalley, P.J.; Benn, J.; Sonksen, P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin. Sci. 1994, 87, 21–29. [Google Scholar] [CrossRef]
- Fleming, T.; Cuny, J.; Nawroth, G.; Djuric, Z.; Humpert, P.M.; Zeier, M.; Bierhaus, A.; Nawroth, P.P. Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia 2012, 55, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, R.; Shuck, S.C.; Chan, Y.S.; Liu, X.; Bates, S.E.; Lim, P.P.; Tamae, D.; Lacoste, S.; O’Connor, T.R.; Termini, J. DNA Advanced Glycation End Products (DNA-AGEs) Are Elevated in Urine and Tissue in an Animal Model of Type 2 Diabetes. Chem. Res. Toxicol. 2017, 30, 689–698. [Google Scholar] [CrossRef]
- Synold, T.; Xi, B.; Wuenschell, G.E.; Tamae, D.; Figarola, J.L.; Rahbar, S.; Termini, J. Advanced glycation end products of DNA: Quantification of N2-(1-Carboxyethyl)-2′-deoxyguanosine in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Chem. Res. Toxicol. 2008, 21, 2148–2155. [Google Scholar] [CrossRef]
- Phillips, S.A.; Mirrlees, D.; Thornalley, P.J. Modification of the glyoxalase system in streptozotocin-induced diabetic rats. Effect of the aldose reductase inhibitor Statil. Biochem. Pharmacol. 1993, 46, 805–811. [Google Scholar] [CrossRef]
- Chou, C.K.; Lee, Y.T.; Chen, S.M.; Hsieh, C.W.; Huang, T.C.; Li, Y.C.; Lee, J.A. Elevated urinary D-lactate levels in patients with diabetes and microalbuminuria. J. Pharm. Biomed. Anal. 2015, 116, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Hanssen, N.M.; van de Waarenburg, M.P.; Jonkers, D.M.; Stehouwer, C.D.; Schalkwijk, C.G. L(+) and D(-) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of L(+) and D(-) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp. Diabetes Res. 2012, 2012, 234812. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.C.; Phillips, S.A.; Thornalley, P.J. The assay of methylglyoxal in biological systems by derivatization with 1,2-diamino-4,5-dimethoxybenzene. Anal. Biochem. 1992, 206, 17–23. [Google Scholar] [CrossRef]
- McLellan, A.C.; Phillips, S.A.; Thornalley, P.J. Fluorimetric assay of D-lactate. Anal. Biochem. 1992, 206, 12–16. [Google Scholar] [CrossRef]
- Christopher, M.M.; Broussard, J.D.; Fallin, C.W.; Drost, N.J.; Peterson, M.E. Increased serum D-lactate associated with diabetic ketoacidosis. Metabolism 1995, 44, 287–290. [Google Scholar] [CrossRef]
- van Eupen, M.G.; Schram, M.T.; Colhoun, H.M.; Hanssen, N.M.; Niessen, H.W.; Tarnow, L.; Parving, H.H.; Rossing, P.; Stehouwer, C.D.; Schalkwijk, C.G. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia 2013, 56, 1845–1855. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Giardino, I.; Torjesen, P.A.; Birkeland, K.I.; Berg, T.J.; Thornalley, P.J.; Brownlee, M.; Hanssen, K.F. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 2003, 52, 163–167. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; van der A, D.L.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: A case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef]
- Fosmark, D.S.; Torjesen, P.A.; Kilhovd, B.K.; Berg, T.J.; Sandvik, L.; Hanssen, K.F.; Agardh, C.D.; Agardh, E. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism 2006, 55, 232–236. [Google Scholar] [CrossRef]
- Phillips, S.A.; Thornalley, P.J. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.; Ponces Freire, A.; Cordeiro, C. The glyoxalase pathway: The first hundred years and beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The Glyoxalase System-New Insights into an Ancient Metabolism. Antioxidants 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 1988, 254, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Cordeiro, C.A.; Ponces Freire, A.M. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 2001, 499, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, X.; Chang, T.; Desai, K.; Wu, L. Attenuation of hypertension development by scavenging methylglyoxal in fructose-treated rats. J. Hypertens. 2008, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Scheijen, J.L.J.M.; Stehouwer, C.D.A.; Wouters, K.; Schalkwijk, C.G. Increased methylglyoxal formation in plasma and tissues during a glucose tolerance test is derived from exogenous glucose. Clin. Sci. 2023, 137, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. 2019, 24, 186–211. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Ford, C.A.; Longerich, L.; Gadag, V.; Wadhawan, S. Role of aldehydes in fructose induced hypertension. Mol. Cell Biochem. 1998, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naomi, N.D.; Ngo, J.; Brouwer-Brolsma, E.M.; Buso, M.E.C.; Soedamah-Muthu, S.S.; Pérez-Rodrigo, C.; Harrold, J.A.; Halford, J.C.G.; Raben, A.; Geleijnse, J.M.; et al. Sugar-sweetened beverages, low/no-calorie beverages, fruit juice and non-alcoholic fatty liver disease defined by fatty liver index: The SWEET project. Nutr. Diabetes 2023, 13, 6. [Google Scholar] [CrossRef]
- Gugliucci, A. Fructose surges damage hepatic adenosyl-monophosphate-dependent kinase and lead to increased lipogenesis and hepatic insulin resistance. Med. Hypotheses 2016, 93, 87–92. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Hernandez-Castillo, C.; Shuck, S.C. Diet and Obesity-Induced Methylglyoxal Production and Links to Metabolic Disease. Chem. Res. Toxicol. 2021, 34, 2424–2440. [Google Scholar] [CrossRef]
- Kalapos, M.P. Where does plasma methylglyoxal originate from? Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef]
- Lyles, G.A.; Chalmers, J. The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem. Pharmacol. 1992, 43, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Qiao, Z.; Sang, S.; Zhang, J.; Zhan, S.; Wu, Z.; Liu, L. Research advances of advanced glycation end products in milk and dairy products: Formation, determination, control strategy and immunometabolism via gut microbiota. Food Chem. 2023, 417, 135861. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food. Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Lim, H.-H.; Shin, H.-S. In-solution derivatization and detection of glyoxal and methylglyoxal in alcoholic beverages and fermented foods by headspace solid-phase microextraction and gas chromatography−mass spectrometry. J. Food Compos. Anal. 2020, 92, 103584. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Gazzani, G. Free α-dicarbonyl compounds in coffee, barley coffee and soy sauce and effects of in vitro digestion. Food Chem. 2014, 164, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic transit of dietary methylglyoxal. J. Agric. Food. Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [CrossRef]
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): Surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14. [Google Scholar] [CrossRef]
- Klöpfer, A.; Spanneberg, R.; Glomb, M.A. Formation of arginine modifications in a model system of Nα-tert-butoxycarbonyl (Boc)-arginine with methylglyoxal. J. Agric. Food Chem. 2011, 59, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005, 280, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5287–5292. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Streeter, M.D.; Spiegel, D.A. Generation and characterization of antibodies against arginine-derived advanced glycation endproducts. Bioorg. Med. Chem. Lett. 2015, 25, 4881–4886. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; Wepy, J.A.; Streeter, M.D.; Kingsley, P.J.; Mitchener, M.M.; Wauchope, O.R.; Beavers, W.N.; Rose, K.L.; Wang, T.; Spiegel, D.A.; et al. Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc. Natl. Acad. Sci. USA 2018, 115, 9228–9233. [Google Scholar] [CrossRef] [PubMed]
- Oya, T.; Hattori, N.; Mizuno, Y.; Miyata, S.; Maeda, S.; Osawa, T.; Uchida, K. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J. Biol. Chem. 1999, 274, 18492–18502. [Google Scholar] [CrossRef] [PubMed]
- Lieuw-a-Fa, M.L.; Schalkwijk, C.G.; Engelse, M.; van Hinsbergh, V.W. Interaction of Nepsilon(carboxymethyl)lysine- and methylglyoxal-modified albumin with endothelial cells and macrophages. Splice variants of RAGE may limit the responsiveness of human endothelial cells to AGEs. Thromb. Haemost. 2006, 95, 320–328. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann. N. Y. Acad. Sci. 2005, 1043, 260–266. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanase, S.; Nakajou, K.; Maruyama, T.; Kragh-Hansen, U.; Otagiri, M. Role of arg-410 and tyr-411 in human serum albumin for ligand binding and esterase-like activity. Biochem. J. 2000, 349, 813–819. [Google Scholar] [CrossRef]
- Faure, P.; Troncy, L.; Lecomte, M.; Wiernsperger, N.; Lagarde, M.; Ruggiero, D.; Halimi, S. Albumin antioxidant capacity is modified by methylglyoxal. Diabetes Metab. 2005, 31, 169–177. [Google Scholar] [CrossRef]
- Abordo, E.A.; Thornalley, P.J. Synthesis and secretion of tumour necrosis factor-alpha by human monocytic THP-1 cells and chemotaxis induced by human serum albumin derivatives modified with methylglyoxal and glucose-derived advanced glycation endproducts. Immunol. Lett. 1997, 58, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Subramaniam, R.; Weiss, M.F.; Monnier, V.M. Methylglyoxal-bovine serum albumin stimulates tumor necrosis factor alpha secretion in RAW 264.7 cells through activation of mitogen-activating protein kinase, nuclear factor kappaB and intracellular reactive oxygen species formation. Arch. Biochem. Biophys. 2003, 409, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.E.; Thornalley, P.J. Induction of synthesis and secretion of interleukin 1 beta in the human monocytic THP-1 cells by human serum albumins modified with methylglyoxal and advanced glycation endproducts. Immunol. Lett. 1996, 50, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 2006, 55, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Sell, D.R.; Strauch, C.; Sun, W.; Lachin, J.M.; Cleary, P.A.; Genuth, S.; DCCT Research Group. The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J. Diabetes Complicat. 2013, 27, 141–149. [Google Scholar] [CrossRef]
- Bose, T.; Bhattacherjee, A.; Banerjee, S.; Chakraborti, A.S. Methylglyoxal-induced modifications of hemoglobin: Structural and functional characteristics. Arch. Biochem. Biophys. 2013, 529, 99–104. [Google Scholar] [CrossRef]
- Lee, J.H.; Samsuzzaman, M.; Park, M.G.; Park, S.J.; Kim, S.Y. Methylglyoxal-derived hemoglobin advanced glycation end products induce apoptosis and oxidative stress in human umbilical vein endothelial cells. Int. J. Biol. Macromol. 2021, 187, 409–421. [Google Scholar] [CrossRef]
- Chen, H.J.; Chen, Y.C.; Hsiao, C.F.; Chen, P.F. Mass Spectrometric Analysis of Glyoxal and Methylglyoxal-Induced Modifications in Human Hemoglobin from Poorly Controlled Type 2 Diabetes Mellitus Patients. Chem. Res. Toxicol. 2015, 28, 2377–2389. [Google Scholar] [CrossRef]
- Jia, X.; Olson, D.J.; Ross, A.R.; Wu, L. Structural and functional changes in human insulin induced by methylglyoxal. FASEB J. 2006, 20, 1555–1557. [Google Scholar] [CrossRef]
- Morcos, M.; Du, X.; Pfisterer, F.; Hutter, H.; Sayed, A.A.; Thornalley, P.; Ahmed, N.; Baynes, J.; Thorpe, S.; Kukudov, G.; et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 2008, 7, 260–269. [Google Scholar] [CrossRef]
- Queisser, M.A.; Yao, D.; Geisler, S.; Hammes, H.P.; Lochnit, G.; Schleicher, E.D.; Brownlee, M.; Preissner, K.T. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 2010, 59, 670–678. [Google Scholar] [CrossRef]
- Folz, R.; Laiteerapong, N. The legacy effect in diabetes: Are there long-term benefits? Diabetologia 2021, 64, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nakamura, S.; Miyazaki, S.; Morita, T.; Suzuki, M.; Pischetsrieder, M.; Niwa, T. N2-carboxyethyl-2′-deoxyguanosine, a DNA glycation marker, in kidneys and aortas of diabetic and uremic patients. Kidney Int. 2006, 69, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Song, J.; Kwon, K.; Jang, S.; Kim, C.; Baek, K.; Kim, J.; Park, C. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 2012, 21, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Smolders, S.; Van Broeckhoven, C. Genetic perspective on the synergistic connection between vesicular transport, lysosomal and mitochondrial pathways associated with Parkinson’s disease pathogenesis. Acta Neuropathol. Commun. 2020, 8, 63. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897. [Google Scholar] [CrossRef]
- Richarme, G.; Liu, C.; Mihoub, M.; Abdallah, J.; Leger, T.; Joly, N.; Liebart, J.C.; Jurkunas, U.V.; Nadal, M.; Bouloc, P.; et al. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science 2017, 357, 208–211. [Google Scholar] [CrossRef]
- Pfaff, D.H.; Fleming, T.; Nawroth, P.; Teleman, A.A. Evidence Against a Role for the Parkinsonism-associated Protein DJ-1 in Methylglyoxal Detoxification. J. Biol. Chem. 2017, 292, 685–690. [Google Scholar] [CrossRef]
- Baba, S.P.; Barski, O.A.; Ahmed, Y.; O’Toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes 2009, 58, 2486–2497. [Google Scholar] [CrossRef]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of Glyoxalase 1 Induces Compensatory Mechanism to Achieve Dicarbonyl Detoxification in Mammalian Schwann Cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects); Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Francisco, F.A.; Barella, L.F.; Silveira, S.D.S.; Saavedra, L.P.J.; Prates, K.V.; Alves, V.S.; Franco, C.C.D.S.; Miranda, R.A.; Ribeiro, T.A.; Tófolo, L.P.; et al. Methylglyoxal treatment in lactating mothers leads to type 2 diabetes phenotype in male rat offspring at adulthood. Eur. J. Nutr. 2018, 57, 477–486. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Desai, K.; Wu, L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc. Res. 2011, 92, 494–503. [Google Scholar] [CrossRef]
- Chang, T.; Wang, R.; Wu, L. Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radic. Biol. Med. 2005, 38, 286–293. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wu, L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem. Pharmacol. 2009, 77, 1709–1716. [Google Scholar] [CrossRef]
- Tsokanos, F.F.; Muley, C.; Khani, S.; Hass, D.; Fleming, T.; Wolff, G.; Bartelt, A.; Nawroth, P.; Herzig, S. Methylglyoxal Drives a Distinct, Nonclassical Macrophage Activation Status. Thromb. Haemost. 2021, 121, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Prantner, D.; Nallar, S.; Richard, K.; Spiegel, D.; Collins, K.D.; Vogel, S.N. Classically activated mouse macrophages produce methylglyoxal that induces a TLR4- and RAGE-independent proinflammatory response. J. Leukoc. Biol. 2021, 109, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wu, L. Accumulation of endogenous methylglyoxal impaired insulin signaling in adipose tissue of fructose-fed rats. Mol. Cell Biochem. 2007, 306, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Wang, R.; Olson, D.J.; Mousseau, D.D.; Ross, A.R.; Wu, L. Modification of Akt1 by methylglyoxal promotes the proliferation of vascular smooth muscle cells. FASEB J. 2011, 25, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, U.; Nagarajan, D. All-Trans Retinoic Acid supplementation prevents cardiac fibrosis and cytokines induced by Methylglyoxal. Glycoconj. J. 2017, 34, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Matafome, P.; Seiça, R. Methylglyoxal further impairs adipose tissue metabolism after partial decrease of blood supply. Arch. Physiol. Biochem. 2013, 119, 209–218. [Google Scholar] [CrossRef]
- Berlanga, J.; Cibrian, D.; Guillén, I.; Freyre, F.; Alba, J.S.; Lopez-Saura, P.; Merino, N.; Aldama, A.; Quintela, A.M.; Triana, M.E.; et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin. Sci. 2005, 109, 83–95. [Google Scholar] [CrossRef]
- Guo, Q.; Mori, T.; Jiang, Y.; Hu, C.; Osaki, Y.; Yoneki, Y.; Sun, Y.; Hosoya, T.; Kawamata, A.; Ogawa, S.; et al. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats. J. Hypertens. 2009, 27, 1664–1671. [Google Scholar] [CrossRef]
- Dhar, A.; Desai, K.M.; Wu, L. Alagebrium attenuates acute methylglyoxal-induced glucose intolerance in Sprague-Dawley rats. Br. J. Pharmacol. 2010, 159, 166–175. [Google Scholar] [CrossRef]
- Nigro, C.; Raciti, G.A.; Leone, A.; Fleming, T.H.; Longo, M.; Prevenzano, I.; Fiory, F.; Mirra, P.; D’Esposito, V.; Ulianich, L.; et al. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia 2014, 57, 1485–1494. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Dhar, I.; Dhar, A.; Wu, L.; Desai, K.M. Methylglyoxal, a reactive glucose metabolite, increases renin angiotensin aldosterone and blood pressure in male Sprague-Dawley rats. Am. J. Hypertens. 2014, 27, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Crisóstomo, J.; Rodrigues, T.; Rodrigues, L.; Sena, C.M.; Pereira, P.; Seiça, R. Methylglyoxal causes structural and functional alterations in adipose tissue independently of obesity. Arch. Physiol. Biochem. 2012, 118, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Hüttl, M.; Markova, I.; Miklankova, D.; Makovicky, P.; Pelikanova, T.; Šeda, O.; Šedová, L.; Malinska, H. Adverse Effects of Methylglyoxal on Transcriptome and Metabolic Changes in Visceral Adipose Tissue in a Prediabetic Rat Model. Antioxidants 2020, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Desai, K.; Clausen, J.T.; Wu, L. Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kidney Int. 2004, 66, 2315–2321. [Google Scholar] [CrossRef]
- Wang, X.; Desai, K.; Chang, T.; Wu, L. Vascular methylglyoxal metabolism and the development of hypertension. J. Hypertens. 2005, 23, 1565–1573. [Google Scholar] [CrossRef]
- Wang, X.; Chang, T.; Jiang, B.; Desai, K.; Wu, L. Attenuation of hypertension development by aminoguanidine in spontaneously hypertensive rats: Role of methylglyoxal. Am. J. Hypertens. 2007, 20, 629–636. [Google Scholar] [CrossRef]
- Mukohda, M.; Okada, M.; Hara, Y.; Yamawaki, H. Methylglyoxal accumulation in arterial walls causes vascular contractile dysfunction in spontaneously hypertensive rats. J. Pharmacol. Sci. 2012, 120, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-Glyoxalase 1 Balance: The Root of Vascular Damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Shamsaldeen, Y.A.; Mackenzie, L.S.; Lione, L.A.; Benham, C.D. Methylglyoxal, A Metabolite Increased in Diabetes is Associated with Insulin Resistance, Vascular Dysfunction and Neuropathies. Curr. Drug Metab. 2016, 17, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mey, J.T.; Haus, J.M. Dicarbonyl Stress and Glyoxalase-1 in Skeletal Muscle: Implications for Insulin Resistance and Type 2 Diabetes. Front. Cardiovasc. Med. 2018, 5, 117. [Google Scholar] [CrossRef]
- Riboulet-Chavey, A.; Pierron, A.; Durand, I.; Murdaca, J.; Giudicelli, J.; Van Obberghen, E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 2006, 55, 1289–1299. [Google Scholar] [CrossRef]
- Engelbrecht, B.; Mattern, Y.; Scheibler, S.; Tschoepe, D.; Gawlowski, T.; Stratmann, B. Methylglyoxal impairs GLUT4 trafficking and leads to increased glucose uptake in L6 myoblasts. Horm. Metab. Res. 2014, 46, 77–84. [Google Scholar] [CrossRef]
- Rodrigues, T.; Matafome, P.; Sereno, J.; Almeida, J.; Castelhano, J.; Gamas, L.; Neves, C.; Gonçalves, S.; Carvalho, C.; Arslanagic, A.; et al. Methylglyoxal-induced glycation changes adipose tissue vascular architecture, flow and expansion, leading to insulin resistance. Sci. Rep. 2017, 7, 1698. [Google Scholar] [CrossRef] [PubMed]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Mirra, P.; Nigro, C.; Prevenzano, I.; Leone, A.; Raciti, G.A.; Formisano, P.; Beguinot, F.; Miele, C. The Destiny of Glucose from a MicroRNA Perspective. Front. Endocrinol. 2018, 9, 46. [Google Scholar] [CrossRef]
- Minjares, M.; Wu, W.; Wang, J.M. Oxidative Stress and MicroRNAs in Endothelial Cells under Metabolic Disorders. Cells 2023, 12, 1341. [Google Scholar] [CrossRef]
- Mirra, P.; Nigro, C.; Prevenzano, I.; Procopio, T.; Leone, A.; Raciti, G.A.; Andreozzi, F.; Longo, M.; Fiory, F.; Beguinot, F.; et al. The role of miR-190a in methylglyoxal-induced insulin resistance in endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 440–449. [Google Scholar] [CrossRef]
- Nigro, C.; Mirra, P.; Prevenzano, I.; Leone, A.; Fiory, F.; Longo, M.; Cabaro, S.; Oriente, F.; Beguinot, F.; Miele, C. miR-214-Dependent Increase of PHLPP2 Levels Mediates the Impairment of Insulin-Stimulated Akt Activation in Mouse Aortic Endothelial Cells Exposed to Methylglyoxal. Int. J. Mol. Sci. 2018, 19, 522. [Google Scholar] [CrossRef] [PubMed]
- Fiory, F.; Lombardi, A.; Miele, C.; Giudicelli, J.; Beguinot, F.; Van Obberghen, E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia 2011, 54, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Xie, S.; Guo, Y.; Zhang, C.; Guan, Y.; Li, C.; Lu, J.; Meng, Q.H. Methylglyoxal Impairs Insulin Secretion of Pancreatic β-Cells through Increased Production of ROS and Mitochondrial Dysfunction Mediated by Upregulation of UCP2 and MAPKs. J. Diabetes Res. 2016, 2016, 2029854. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef]
- Ozdemir, A.M.; Hopfer, U.; Rosca, M.V.; Fan, X.J.; Monnier, V.M.; Weiss, M.F. Effects of advanced glycation end product modification on proximal tubule epithelial cell processing of albumin. Am. J. Nephrol. 2008, 28, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Nawroth, P.P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef]
- Jack, M.M.; Ryals, J.M.; Wright, D.E. Characterisation of glyoxalase I in a streptozocin-induced mouse model of diabetes with painful and insensate neuropathy. Diabetologia 2011, 54, 2174–2182. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Eringa, E.C.; Serne, E.H.; Meijer, R.I.; Schalkwijk, C.G.; Houben, A.J.; Stehouwer, C.D.; Smulders, Y.M.; van Hinsbergh, V.W. Endothelial dysfunction in (pre)diabetes: Characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev. Endocr. Metab. Disord. 2013, 14, 39–48. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D. Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clin. Sci. 2005, 109, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Micali, L.R.; Wouters, K. Advanced glycation endproducts in diabetes-related macrovascular complications: Focus on methylglyoxal. Trends Endocrinol. Metab. 2023, 34, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Ebara, T.; Conde, K.; Kako, Y.; Liu, Y.; Xu, Y.; Ramakrishnan, R.; Goldberg, I.J.; Shachter, N.S. Delayed catabolism of apoB-48 lipoproteins due to decreased heparan sulfate proteoglycan production in diabetic mice. J. Clin. Investig. 2000, 105, 1807–1818. [Google Scholar] [CrossRef]
- Koya, D.; Jirousek, M.R.; Lin, Y.W.; Ishii, H.; Kuboki, K.; King, G.L. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J. Clin. Investig. 1997, 100, 115–126. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Du, X.; Edelstein, D.; Obici, S.; Higham, N.; Zou, M.H.; Brownlee, M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J. Clin. Investig. 2006, 116, 1071–1080. [Google Scholar] [CrossRef]
- Dhar, A.; Dhar, I.; Desai, K.M.; Wu, L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br. J. Pharmacol. 2010, 161, 1843–1856. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef]
- Ravi, R.; Ragavachetty Nagaraj, N.; Subramaniam Rajesh, B. Effect of advanced glycation end product on paraoxonase 2 expression: Its impact on endoplasmic reticulum stress and inflammation in HUVECs. Life Sci. 2020, 246, 117397. [Google Scholar] [CrossRef]
- Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Investig. 1998, 101, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Engelbrecht, B.; Espelage, B.C.; Klusmeier, N.; Tiemann, J.; Gawlowski, T.; Mattern, Y.; Eisenacher, M.; Meyer, H.E.; Rabbani, N.; et al. Glyoxalase 1-knockdown in human aortic endothelial cells—Effect on the proteome and endothelial function estimates. Sci. Rep. 2016, 6, 37737. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, N.; Abe, M.; Souma, T.; Tanemoto, M.; Abe, T.; Nakayama, M.; Ito, S. Methylglyoxal augments intracellular oxidative stress in human aortic endothelial cells. Free Radic. Res. 2010, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Tatsunami, R.; Sato, K.; Takahashi, K.; Hao, Z.; Tampo, Y. Methylglyoxal has deleterious effects on thioredoxin in human aortic endothelial cells. Environ. Toxicol. Pharmacol. 2012, 34, 117–126. [Google Scholar] [CrossRef]
- Brouwers, O.; Teerlink, T.; van Bezu, J.; Barto, R.; Stehouwer, C.D.; Schalkwijk, C.G. Methylglyoxal and methylglyoxal-arginine adducts do not directly inhibit endothelial nitric oxide synthase. Ann. N. Y. Acad. Sci. 2008, 1126, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, L.; Zhou, S.; Zhu, M.; Wang, B. N-acetylcysteine inhibits atherosclerosis by correcting glutathione-dependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice. Mol. Med. Rep. 2021, 23, 201. [Google Scholar] [CrossRef]
- Su, Y.; Qadri, S.M.; Wu, L.; Liu, L. Methylglyoxal modulates endothelial nitric oxide synthase-associated functions in EA.hy926 endothelial cells. Cardiovasc. Diabetol. 2013, 12, 134. [Google Scholar] [CrossRef]
- Chu, P.; Han, G.; Ahsan, A.; Sun, Z.; Liu, S.; Zhang, Z.; Sun, B.; Song, Y.; Lin, Y.; Peng, J.; et al. Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-κB pathway. Vascul. Pharmacol. 2017, 91, 26–35. [Google Scholar] [CrossRef]
- Braun, J.D.; Pastene, D.O.; Breedijk, A.; Rodriguez, A.; Hofmann, B.B.; Sticht, C.; von Ochsenstein, E.; Allgayer, H.; van den Born, J.; Bakker, S.; et al. Methylglyoxal down-regulates the expression of cell cycle associated genes and activates the p53 pathway in human umbilical vein endothelial cells. Sci. Rep. 2019, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rodriguez-Niño, A.; Pastene, D.O.; Pallavi, P.; van den Born, J.; Bakker, S.J.L.; Krämer, B.K.; Yard, B.A. Methylglyoxal induces p53 activation and inhibits mTORC1 in human umbilical vein endothelial cells. Sci. Rep. 2021, 11, 8004. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Navarrete Santos, A.; Jacobs, K.; Simm, A.; Glaubitz, N.; Horstkorte, R.; Hofmann, B. Dicarbonyls induce senescence of human vascular endothelial cells. Mech. Ageing Dev. 2017, 166, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hall, L.M.; Kujawa, M.; Li, H.; Zhang, X.; O’Meara, M.; Ichinose, T.; Wang, J.M. Methylglyoxal triggers human aortic endothelial cell dysfunction via modulation of the KATP/MAPK pathway. Am. J. Physiol. Cell. Physiol. 2019, 317, C68–C81. [Google Scholar] [CrossRef]
- Liu, H.; Yu, S.; Zhang, H.; Xu, J. Angiogenesis impairment in diabetes: Role of methylglyoxal-induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS ONE 2012, 7, e46720. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Giacco, F.; Maeda, K.; Blackburn, N.J.; Brownlee, M.; Milne, R.W.; Suuronen, E.J. Methylglyoxal-Induced Endothelial Cell Loss and Inflammation Contribute to the Development of Diabetic Cardiomyopathy. Diabetes 2016, 65, 1699–1713. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.A.; Shin, Y.J.; Kim, H.; Majid, A.; Bae, O.N. Methylglyoxal induced advanced glycation end products (AGE)/receptor for AGE (RAGE)-mediated angiogenic impairment in bone marrow-derived endothelial progenitor cells. J. Toxicol. Environ. Health A 2018, 81, 266–277. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, E.A.; Park, H.J.; Sung, E.G.; Song, I.H.; Kim, J.Y.; Woo, C.H.; Doh, K.O.; Kim, K.H.; Lee, T.J. Methylglyoxal-induced apoptosis is dependent on the suppression of c-FLIPL expression via down-regulation of p65 in endothelial cells. J. Cell. Mol. Med. 2017, 21, 2720–2731. [Google Scholar] [CrossRef]
- Brouwers, O.; Yu, L.; Niessen, P.; Slenter, J.; Jaspers, K.; Wagenaar, A.; Post, M.; Miyata, T.; Backes, W.; Stehouwer, C.; et al. Glyoxalase-1 overexpression partially prevents diabetes-induced impaired arteriogenesis in a rat hindlimb ligation model. Glycoconj. J. 2016, 33, 627–630. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Longo, M.; Prevenzano, I.; Fleming, T.H.; Nicolò, A.; Parrillo, L.; Spinelli, R.; Formisano, P.; Nawroth, P.P.; et al. Methylglyoxal accumulation de-regulates HoxA5 expression, thereby impairing angiogenesis in glyoxalase 1 knock-down mouse aortic endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 73–85. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Geoffrion, M.; Kuraitis, D.; McBane, J.E.; Lochhead, M.; Vanderhyden, B.C.; Korbutt, G.S.; Milne, R.W.; Suuronen, E.J. Glyoxalase-1 overexpression in bone marrow cells reverses defective neovascularization in STZ-induced diabetic mice. Cardiovasc. Res. 2014, 101, 306–316. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.; Miyata, T.; Østergaard, J.A.; Flyvbjerg, A.; Peutz-Kootstra, C.J.; Sieber, J.; Mundel, P.H.; Brownlee, M.; Janssen, B.J.; et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 2014, 57, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Mukohda, M.; Morita, T.; Okada, M.; Hara, Y.; Yamawaki, H. Long-term methylglyoxal treatment causes endothelial dysfunction of rat isolated mesenteric artery. J. Vet. Med. Sci. 2013, 75, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Haenen, G.; Miyata, T.; Brownlee, M.; Stehouwer, C.D.; De Mey, J.G.; Schalkwijk, C.G. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia 2010, 53, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Pickering, R.J.; Tsorotes, D.; Huet, O.; Cooper, M.E.; Jandeleit-Dahm, K.; Thomas, M.C. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes 2014, 63, 3915–3925. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; de Vos-Houben, J.M.; Niessen, P.M.; Miyata, T.; van Nieuwenhoven, F.; Janssen, B.J.; Hageman, G.; Stehouwer, C.D.; Schalkwijk, C.G. Mild oxidative damage in the diabetic rat heart is attenuated by glyoxalase-1 overexpression. Int. J. Mol. Sci. 2013, 14, 15724–15739. [Google Scholar] [CrossRef] [PubMed]
- Geoffrion, M.; Du, X.; Irshad, Z.; Vanderhyden, B.C.; Courville, K.; Sui, G.; D’Agati, V.D.; Ott-Braschi, S.; Rabbani, N.; Thornalley, P.J.; et al. Differential effects of glyoxalase 1 overexpression on diabetic atherosclerosis and renal dysfunction in streptozotocin-treated, apolipoprotein E-deficient mice. Physiol. Rep. 2014, 2, e12043. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Brouwers, O.; Gijbels, M.J.; Wouters, K.; Wijnands, E.; Cleutjens, J.P.; De Mey, J.G.; Miyata, T.; Biessen, E.A.; Stehouwer, C.D.; et al. Glyoxalase 1 overexpression does not affect atherosclerotic lesion size and severity in ApoE-/- mice with or without diabetes. Cardiovasc. Res. 2014, 104, 160–170. [Google Scholar] [CrossRef]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Engelen, L.; Ferreira, I.; Scheijen, J.L.; Huijberts, M.S.; van Greevenbroek, M.M.; van der Kallen, C.J.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.; et al. Plasma levels of advanced glycation endproducts Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: The Hoorn and CODAM studies. J. Clin. Endocrinol. Metab. 2013, 98, E1369–E1373. [Google Scholar] [CrossRef]
- Semba, R.D.; Bandinelli, S.; Sun, K.; Guralnik, J.M.; Ferrucci, L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J. Am. Geriatr. Soc. 2009, 57, 1874–1880. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J.M.; Fried, L.P. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin. Exp. Res. 2009, 21, 182–190. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Hanssen, K.F.; Laakso, M. Increased serum levels of methylglyoxal-derived hydroimidazolone-AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis 2009, 205, 590–594. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Birkeland, K.I.; Berg, T.J.; Hanssen, K.F.; Laakso, M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: A population-based 18-year follow-up study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 815–820. [Google Scholar] [CrossRef]
- Busch, M.; Franke, S.; Wolf, G.; Brandstädt, A.; Ott, U.; Gerth, J.; Hunsicker, L.G.; Stein, G.; Collaborative Study Group. The advanced glycation end product N(epsilon)-carboxymethyllysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension. Am. J. Kidney Dis. 2006, 48, 571–579. [Google Scholar] [CrossRef]
- Schwedler, S.B.; Metzger, T.; Schinzel, R.; Wanner, C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002, 62, 301–310. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Scheijen, J.L.J.M.; Jorsal, A.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D.A.; Schalkwijk, C.G. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease in Individuals With Type 1 Diabetes: A 12-Year Follow-up Study. Diabetes 2017, 66, 2278–2283. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Westerink, J.; Scheijen, J.L.J.M.; van der Graaf, Y.; Stehouwer, C.D.A.; Schalkwijk, C.G.; SMART Study Group. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease and Mortality in Individuals With Type 2 Diabetes. Diabetes Care 2018, 41, 1689–1695. [Google Scholar] [CrossRef]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Scheijen, J.L.J.M.; Houben, A.J.H.M.; van de Waarenburg, M.; Berendschot, T.T.J.M.; Webers, C.A.B.; Reesink, K.D.; van Greevenbroek, M.M.J.; van der Kallen, C.; Schaper, N.C.; et al. Fasting and post-oral-glucose-load levels of methylglyoxal are associated with microvascular, but not macrovascular, disease in individuals with and without (pre)diabetes: The Maastricht Study. Diabetes Metab. 2021, 47, 101148. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Wouters, K.; Huijberts, M.S.; Gijbels, M.J.; Sluimer, J.C.; Scheijen, J.L.; Heeneman, S.; Biessen, E.A.; Daemen, M.J.; Brownlee, M.; et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur. Heart J. 2014, 35, 1137–1146. [Google Scholar] [CrossRef]
- Baidoshvili, A.; Niessen, H.W.; Stooker, W.; Huybregts, R.A.; Hack, C.E.; Rauwerda, J.A.; Meijer, C.J.; Eijsman, L.; van Hinsbergh, V.W.; Schalkwijk, C.G. N(omega)-(carboxymethyl)lysine depositions in human aortic heart valves: Similarities with atherosclerotic blood vessels. Atherosclerosis 2004, 174, 287–292. [Google Scholar] [CrossRef]
- Varona, J.F.; Ortiz-Regalón, R.; Sánchez-Vera, I.; López-Melgar, B.; García-Durango, C.; Castellano Vázquez, J.M.; Solís, J.; Fernández-Friera, L.; Vidal-Vanaclocha, F. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch. Med. Res. 2019, 50, 20–28. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Keller, T.T.; Wareham, N.J.; Luben, R.; Bingham, S.A.; Day, N.E.; Sandhu, M.S.; Jukema, J.W.; Kastelein, J.J.; Hack, C.E.; et al. Serum levels of type II secretory phospholipase A2 and the risk of future coronary artery disease in apparently healthy men and women: The EPIC-Norfolk Prospective Population Study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 839–846. [Google Scholar] [CrossRef]
- Berge, K.; Aengevaeren, V.L.; Mosterd, A.; Velthuis, B.K.; Lyngbakken, M.N.; Omland, T.; Schalkwijk, C.G.; Eijsvogels, T.M.H. Plasma Advanced Glycation End Products and Dicarbonyl Compounds Are Not Associated with Coronary Atherosclerosis in Athletes. Med. Sci. Sports Exerc. 2023, 55, 1143–1150. [Google Scholar] [CrossRef]
- Kerkeni, M.; Weiss, I.S.; Jaisson, S.; Dandana, A.; Addad, F.; Gillery, P.; Hammami, M. Increased serum concentrations of pentosidine are related to presence and severity of coronary artery disease. Thromb. Res. 2014, 134, 633–638. [Google Scholar] [CrossRef]
- Mäkinen, V.P.; Civelek, M.; Meng, Q.; Zhang, B.; Zhu, J.; Levian, C.; Huan, T.; Segrè, A.V.; Ghosh, S.; Vivar, J.; et al. Integrative genomics reveals novel molecular pathways and gene networks for coronary artery disease. PLoS Genet. 2014, 10, e1004502. [Google Scholar] [CrossRef]
- Ren, J.; Bi, Y.; Sowers, J.R.; Hetz, C.; Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021, 18, 499–521. [Google Scholar] [CrossRef]
- Irshad, Z.; Xue, M.; Ashour, A.; Larkin, J.R.; Thornalley, P.J.; Rabbani, N. Activation of the unfolded protein response in high glucose treated endothelial cells is mediated by methylglyoxal. Sci. Rep. 2019, 9, 7889. [Google Scholar] [CrossRef]
- Kırça, M.; Yeşilkaya, A. Methylglyoxal stimulates endoplasmic reticulum stress in vascular smooth muscle cells. J. Recept. Signal Transduct. Res. 2022, 42, 279–284. [Google Scholar] [CrossRef]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef]
- Natalucci, S.; Ruggeri, P.; Cogo, C.E.; Picchio, V.; Burattini, R. Insulin sensitivity and glucose effectiveness estimated by the minimal model technique in spontaneously hypertensive and normal rats. Exp. Physiol. 2000, 85, 775–781. [Google Scholar] [CrossRef]
- Natalucci, S.; Ruggeri, P.; Cogo, C.E.; Picchio, V.; Brunori, A.; Burattini, R. Age-related analysis of glucose metabolism in spontaneously hypertensive and normotensive rats. Exp. Physiol. 2003, 88, 399–404. [Google Scholar] [CrossRef]
- Wu, L.; Juurlink, B.H. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension 2002, 39, 809–814. [Google Scholar] [CrossRef]
- Wang, H.; Meng, Q.H.; Chang, T.; Wu, L. Fructose-induced peroxynitrite production is mediated by methylglyoxal in vascular smooth muscle cells. Life Sci. 2006, 79, 2448–2454. [Google Scholar] [CrossRef]
- Baden, T.; Yamawaki, H.; Saito, K.; Mukohda, M.; Okada, M.; Hara, Y. Telmisartan inhibits methylglyoxal-mediated cell death in human vascular endothelium. Biochem. Biophys. Res. Commun. 2008, 373, 253–257. [Google Scholar] [CrossRef]
- Mukohda, M.; Yamawaki, H.; Nomura, H.; Okada, M.; Hara, Y. Methylglyoxal inhibits smooth muscle contraction in isolated blood vessels. J. Pharmacol. Sci. 2009, 109, 305–310. [Google Scholar] [CrossRef]
- Jacobson, R.; Mignemi, N.; Rose, K.; O’Rear, L.; Sarilla, S.; Hamm, H.E.; Barnett, J.V.; Verhamme, I.M.; Schoenecker, J. The hyperglycemic byproduct methylglyoxal impairs anticoagulant activity through covalent adduction of antithrombin III. Thromb. Res. 2014, 134, 1350–1357. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Zou, Y.L.; Guo, S.D. Low-density lipoprotein particles in atherosclerosis. Front. Physiol. 2022, 13, 931931. [Google Scholar] [CrossRef]
- Rabbani, N.; Godfrey, L.; Xue, M.; Shaheen, F.; Geoffrion, M.; Milne, R.; Thornalley, P.J. Glycation of LDL by methylglyoxal increases arterial atherogenicity: A possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes 2011, 60, 1973–1980. [Google Scholar] [CrossRef]
- Rabbani, N.; Chittari, M.V.; Bodmer, C.W.; Zehnder, D.; Ceriello, A.; Thornalley, P.J. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010, 59, 1038–1045. [Google Scholar] [CrossRef]
- Lankin, V.; Konovalova, G.; Tikhaze, A.; Shumaev, K.; Kumskova, E.; Viigimaa, M. The initiation of free radical peroxidation of low-density lipoproteins by glucose and its metabolite methylglyoxal: A common molecular mechanism of vascular wall injure in atherosclerosis and diabetes. Mol. Cell. Biochem. 2014, 395, 241–252. [Google Scholar] [CrossRef]
- Godfrey, L.; Yamada-Fowler, N.; Smith, J.; Thornalley, P.J.; Rabbani, N. Arginine-directed glycation and decreased HDL plasma concentration and functionality. Nutr. Diabetes 2014, 4, e134. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Tsukushi, T.; Munekata, S.; Akahoshi, T.; Moriya, T.; Ogawa, Z. Semiquantitative analysis of apolipoprotein A-I modified by advanced glycation end products in diabetes mellitus. J. Clin. Lab. Anal. 2013, 27, 231–236. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Wu, Z.; Pan, D.; Yan, N.; Liu, L. Cereal polyphenols inhibition mechanisms on advanced glycation end products and regulation on type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2023. ahead-of-print. [Google Scholar] [CrossRef]
- Miller, A.G.; Tan, G.; Nagaraj, R.H.; Cooper, M.E.; Wilkinson-Berka, J.L. Candesartan Attenuates Vascular Injury in Diabetic Retinopathy by Restoring Glyoxalase I Function. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4806. [Google Scholar]
- Miller, A.G.; Tan, G.; Binger, K.J.; Pickering, R.J.; Thomas, M.C.; Nagaraj, R.H.; Cooper, M.E.; Wilkinson-Berka, J.L. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes 2010, 59, 3208–3215. [Google Scholar] [CrossRef]
- Oh, S.; Ahn, H.; Park, H.; Lee, J.I.; Park, K.Y.; Hwang, D.; Lee, S.; Son, K.H.; Byun, K. The attenuating effects of pyridoxamine on adipocyte hypertrophy and inflammation differ by adipocyte location. J. Nutr. Biochem. 2019, 72, 108173. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Reversal of Insulin Resistance in Overweight and Obese Subjects by trans-Resveratrol and Hesperetin Combination-Link to Dysglycemia, Blood Pressure, Dyslipidemia, and Low-Grade Inflammation. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Salazar, J.; Navarro, C.; Ortega, Á.; Nava, M.; Morillo, D.; Torres, W.; Hernández, M.; Cabrera, M.; Angarita, L.; Ortiz, R.; et al. Advanced Glycation End Products: New Clinical and Molecular Perspectives. Int. J. Environ. Res. Public. Health 2021, 18, 7236. [Google Scholar] [CrossRef]
- Van den Eynde, M.D.G.; Geleijnse, J.M.; Scheijen, J.L.J.M.; Hanssen, N.M.J.; Dower, J.I.; Afman, L.A.; Stehouwer, C.D.A.; Hollman, P.C.H.; Schalkwijk, C.G. Quercetin, but Not Epicatechin, Decreases Plasma Concentrations of Methylglyoxal in Adults in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial with Pure Flavonoids. J. Nutr. 2018, 148, 1911–1916. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yoon, Y.W.; Lee, B.K.; Kwon, H.M.; Hwang, K.C.; Kim, M.; Chang, W.; Hong, B.K.; Lee, Y.H.; Park, S.J.; et al. Potential role of HMG CoA reductase inhibitor on oxidative stress induced by advanced glycation endproducts in vascular smooth muscle cells of diabetic vasculopathy. Exp. Mol. Med. 2009, 41, 802–811. [Google Scholar] [CrossRef]
- Recabarren-Leiva, D.; Burgos, C.F.; Hernández, B.; Garcïa-García, F.J.; Castro, R.I.; Guzman, L.; Fuentes, E.; Palomo, I.; Alarcón, M. Effects of the age/rage axis in the platelet activation. Int. J. Biol. Macromol. 2021, 166, 1149–1161. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Q.; Chen, F.; Pang, J.; Pan, C.; Xu, F.; Chen, Y. Fundamental Mechanisms of the Cell Death Caused by Nitrosative Stress. Front. Cell Dev. Biol. 2021, 9, 742483. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: A novel therapeutic strategy for diabetic vascular complications. Expert. Opin. Investig. Drugs 2008, 17, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Quade-Lyssy, P.; Kanarek, A.M.; Baiersdörfer, M.; Postina, R.; Kojro, E. Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. J. Lipid Res. 2013, 54, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Toprak, C.; Yigitaslan, S. Alagebrium and Complications of Diabetes Mellitus. Eurasian J. Med. 2019, 51, 285–292. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Tikellis, C.; Pickering, R.J.; Dragoljevic, D.; Lee, M.K.S.; Block, T.; Scheijen, J.L.; Wouters, K.; Miyata, T.; Cooper, M.E.; et al. Pyridoxamine prevents increased atherosclerosis by intermittent methylglyoxal spikes in the aortic arches of ApoE−/− mice. Biomed. Pharmacother. 2023, 158, 114211. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol. 2004, 24, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kinsky, O.R.; Hargraves, T.L.; Anumol, T.; Jacobsen, N.E.; Dai, J.; Snyder, S.A.; Monks, T.J.; Lau, S.S. Metformin Scavenges Methylglyoxal To Form a Novel Imidazolinone Metabolite in Humans. Chem. Res. Toxicol. 2016, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, Y.; Yamagishi, S.; Takeuchi, M.; Ishida, S.; Jinnouchi, Y.; Jinnouchi, J.; Imaizumi, T. Atorvastatin decreases serum levels of advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin. Exp. Med. 2006, 6, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Scharnagl, H.; Stojakovic, T.; Winkler, K.; Rosinger, S.; März, W.; Boehm, B.O. The HMG-CoA reductase inhibitor cerivastatin lowers advanced glycation end products in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2007, 115, 372–375. [Google Scholar] [CrossRef]
- Contreras, C.L.; Guzman-Rosiles, I.; Del Castillo, D.; Gomez-Ojeda, A.; Garay-Sevilla, M.E. Advanced glycation end products (AGEs) and sRAGE levels after benfotiamine treatment in diabetes mellitus type 2. FASEB J. 2017, 31, 646.32. [Google Scholar] [CrossRef]
- Van den Eynde, M.D.G.; Houben, A.J.H.M.; Scheijen, J.L.J.M.; Linkens, A.M.A.; Niessen, P.M.; Simons, N.; Hanssen, N.M.J.; Kusters, Y.H.A.M.; Eussen, S.J.M.P.; Miyata, T.; et al. Pyridoxamine reduces methylglyoxal and markers of glycation and endothelial dysfunction, but does not improve insulin sensitivity or vascular function in abdominally obese individuals: A randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2023, 25, 1280–1291. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I.; Scheijen, J.L.J.M.; Ahles, S.; Vangrieken, P.; Schalkwijk, C.G. A Citrus and Pomegranate Complex Reduces Methylglyoxal in Healthy Elderly Subjects: Secondary Analysis of a Double-Blind Randomized Cross-Over Clinical Trial. Int. J. Mol. Sci. 2023, 24, 13168. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Colzani, M.; De Maddis, D.; Casali, G.; Carini, M.; Vistoli, G.; Aldini, G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A Comparative Study. ChemMedChem 2016, 11, 1778–1789. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Potential of Vasoprotectives to Inhibit Non-Enzymatic Protein Glycation, and Reactive Carbonyl and Oxygen Species Uptake. Int. J. Mol. Sci. 2021, 22, 10026. [Google Scholar] [CrossRef] [PubMed]
- Voziyan, P.A.; Metz, T.O.; Baynes, J.W.; Hudson, B.G. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J. Biol. Chem. 2002, 277, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero-Lopez, D.; Lecomte, M.; Moinet, G.; Patereau, G.; Lagarde, M.; Wiernsperger, N. Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation. Biochem. Pharmacol. 1999, 58, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Kiho, T.; Kato, M.; Usui, S.; Hirano, K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin. Chim. Acta 2005, 358, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Adeshara, K.; Tupe, R. Antiglycation and cell protective actions of metformin and glipizide in erythrocytes and monocytes. Mol. Biol. Rep. 2016, 43, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.S.; Wortmann, M.; Fleming, T.H.; Nawroth, P.P.; Bruckner, T.; Böckler, D.; Hakimi, M. Effect of metformin treatment in patients with type 2 diabetes with respect to glyoxalase 1 activity in atherosclerotic lesions. Vasa 2019, 48, 186–192. [Google Scholar] [CrossRef]
- Rahbar, S.; Natarajan, R.; Yerneni, K.; Scott, S.; Gonzales, N.; Nadler, J.L. Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin. Chim. Acta 2000, 301, 65–77. [Google Scholar] [CrossRef]
- Qais, F.A.; Sarwar, T.; Ahmad, I.; Khan, R.A.; Shahzad, S.A.; Husain, F.M. Glyburide inhibits non-enzymatic glycation of HSA: An approach for the management of AGEs associated diabetic complications. Int. J. Biol. Macromol. 2021, 169, 143–152. [Google Scholar] [CrossRef]
- Li, W.; Ota, K.; Nakamura, J.; Naruse, K.; Nakashima, E.; Oiso, Y.; Hamada, Y. Antiglycation effect of gliclazide on in vitro AGE formation from glucose and methylglyoxal. Exp. Biol. Med. 2008, 233, 176–179. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, J.R.; Choi, H.C. Gliclazide, a KATP channel blocker, inhibits vascular smooth muscle cell proliferation through the CaMKKβ-AMPK pathway. Vascul. Pharmacol. 2018, 102, 21–28. [Google Scholar] [CrossRef]
- Koyama, H.; Tanaka, S.; Monden, M.; Shoji, T.; Morioka, T.; Fukumoto, S.; Mori, K.; Emoto, M.; Shoji, T.; Fukui, M.; et al. Comparison of effects of pioglitazone and glimepiride on plasma soluble RAGE and RAGE expression in peripheral mononuclear cells in type 2 diabetes: Randomized controlled trial (PioRAGE). Atherosclerosis 2014, 234, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Walcher, D.; Ivanova, N.; Rautzenberg, K.; Jung, A.; Friedl, R.; Hombach, V.; de Caterina, R.; Basta, G.; Wautier, M.P.; et al. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes 2004, 53, 2662–2668. [Google Scholar] [CrossRef]
- Liu, Y.; Park, J.M.; Chang, K.H.; Huh, H.J.; Lee, K.; Lee, M.Y. AMP-Activated Protein Kinase Mediates the Antiplatelet Effects of the Thiazolidinediones Rosiglitazone and Pioglitazone. Mol. Pharmacol. 2016, 89, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Adeshara, K.A.; Agrawal, S.B.; Gaikwad, S.M.; Tupe, R.S. Pioglitazone inhibits advanced glycation induced protein modifications and down-regulates expression of RAGE and NF-κB in renal cells. Int. J. Biol. Macromol. 2018, 119, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Oz Gul, O.; Tuncel, E.; Yilmaz, Y.; Ulukaya, E.; Gul, C.B.; Kiyici, S.; Oral, A.Y.; Guclu, M.; Ersoy, C.; Imamoglu, S. Comparative effects of pioglitazone and rosiglitazone on plasma levels of soluble receptor for advanced glycation end products in type 2 diabetes mellitus patients. Metabolism 2010, 59, 64–69. [Google Scholar] [CrossRef]
- Jakuš, V.; Hrnciarová, M.; Cársky, J.; Krahulec, B.; Rietbrock, N. Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity. Life Sci. 1999, 65, 1991–1993. [Google Scholar] [CrossRef]
- Miyata, T.; van Ypersele de Strihou, C.; Ueda, Y.; Ichimori, K.; Inagi, R.; Onogi, H.; Ishikawa, N.; Nangaku, M.; Kurokawa, K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: Biochemical mechanisms. J. Am. Soc. Nephrol. 2002, 13, 2478–2487. [Google Scholar] [CrossRef]
- Nangaku, M.; Miyata, T.; Sada, T.; Mizuno, M.; Inagi, R.; Ueda, Y.; Ishikawa, N.; Yuzawa, H.; Koike, H.; van Ypersele de Strihou, C.; et al. Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J. Am. Soc. Nephrol. 2003, 14, 1212–1222. [Google Scholar] [CrossRef]
- Sobal, G.; Menzel, E.J.; Sinzinger, H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochem. Pharmacol. 2001, 61, 373–379. [Google Scholar] [CrossRef]
- Brown, B.E.; Mahroof, F.M.; Cook, N.L.; van Reyk, D.M.; Davies, M.J. Hydrazine compounds inhibit glycation of low-density lipoproteins and prevent the in vitro formation of model foam cells from glycolaldehyde-modified low-density lipoproteins. Diabetologia 2006, 49, 775–783. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis 1998, 138, 271–280. [Google Scholar] [CrossRef]
- Lu, L.; Peng, W.H.; Wang, W.; Wang, L.J.; Chen, Q.J.; Shen, W.F. Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats. J. Zhejiang Univ. Sci. B 2011, 12, 652–659. [Google Scholar] [CrossRef]
- Hwang, A.R.; Nam, J.O.; Kang, Y.J. Fluvastatin inhibits advanced glycation end products-induced proliferation, migration, and extracellular matrix accumulation in vascular smooth muscle cells by targeting connective tissue growth factor. Korean J. Physiol. Pharmacol. 2018, 22, 193–201. [Google Scholar] [CrossRef]
- Fukuda, K.; Matsumura, T.; Senokuchi, T.; Ishii, N.; Kinoshita, H.; Yamada, S.; Murakami, S.; Nakao, S.; Motoshima, H.; Kondo, T.; et al. Statins meditate anti-atherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-γ activation. Biochem. Biophys. Res. Commun. 2015, 457, 23–30. [Google Scholar] [CrossRef]
- Hwang, A.R.; Han, J.H.; Lim, J.H.; Kang, Y.J.; Woo, C.H. Fluvastatin inhibits AGE-induced cell proliferation and migration via an ERK5-dependent Nrf2 pathway in vascular smooth muscle cells. PLoS ONE 2017, 12, e0178278. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yamagishi, S.; Matsui, T.; Ohta, K.; Tanoue, R.; Takeuchi, M.; Ueda, S.; Nakamura, K.; Okuda, S. Pravastatin inhibits advanced glycation end products (AGEs)-induced proximal tubular cell apoptosis and injury by reducing receptor for AGEs (RAGE) level. Metabolism 2012, 61, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; De Marco, F.; Di Domenico, F.; Coccia, R.; Lusini, M.; Barbato, R.; Covino, E.; Chello, M. Simvastatin attenuates the endothelial pro-thrombotic shift in saphenous vein grafts induced by Advanced glycation endproducts. Thromb. Res. 2014, 133, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cai, X.; Hao, M.; Liu, X.; Chen, Z.; Li, H.; Liu, J.; Liao, Y.; Fu, H.; Chen, H.; et al. Calcium Dobesilate (CaD) Attenuates High Glucose and High Lipid-Induced Impairment of Sarcoplasmic Reticulum Calcium Handling in Cardiomyocytes. Front. Cardiovasc. Med. 2021, 8, 637021. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.L.; Seres, A.I.; Carneiro, A.M.; Stur, M.; Zourdani, A.; Caillon, P.; Cunha-Vaz, J.G.; DX-Retinopathy Study Group. Effect of calcium dobesilate on progression of early diabetic retinopathy: A randomised double-blind study. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Feng, J.; Sun, L.; Suo, X.; Yao, L.; LI, Z.; Wang, Y.; Zhou, J.; Wang, L. Aristolochic acid-induced endothelial cell injury and the mechanism of calcium dobesilate antagonism. J. Chin. Physician 2009, 11, 913–916. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, C.; Li, S.; Shao, X.; Mou, S.; Ni, Z. Diabetic Nephropathy Can Be Treated with Calcium Dobesilate by Alleviating the Chronic Inflammatory State and Improving Endothelial Cell Function. Cell Physiol. Biochem. 2018, 51, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Liu, Y.J.; Huang, Y.; Yu, H.J.; Yuan, S.; Tang, B.W.; Wang, P.G.; He, Q.Q. Dietary total flavonoids intake and risk of mortality from all causes and cardiovascular disease in the general population: A systematic review and meta-analysis of cohort studies. Mol. Nutr. Food Res. 2017, 61, 1601003. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of Quercetin and Its Methylglyoxal Adducts on the Formation of α-Dicarbonyl Compounds in a Lysine/Glucose Model System. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Holovko, O.O.; Dmytrenko, O.P.; Lesiuk, A.I.; Kulish, M.P.; Pavlenko, O.L.; Naumenko, A.P.; Pinchuk-Rugal, T.M.; Kaniuk, M.I.; Veklich, T.O. Mechanisms of the interaction of bovine serum albumin with quercetin. Mol. Cryst. Liq. Cryst. 2023, 701, 59–71. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2017, 20, 68–77. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am J Epidemiol 2017, 185, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef]
- Huxley, R.R.; Neil, H.A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, H.; He, B. Dietary flavonoids intake and the risk of coronary heart disease: A dose-response meta-analysis of 15 prospective studies. Thromb. Res. 2015, 135, 459–463. [Google Scholar] [CrossRef]
- van Boekel, M.A.; van den Bergh, P.J.; Hoenders, H.J. Glycation of human serum albumin: Inhibition by Diclofenac. Biochim. Biophys. Acta 1992, 1120, 201–204. [Google Scholar] [CrossRef]
- Rendell, M.; Nierenberg, J.; Brannan, C.; Valentine, J.L.; Stephen, P.M.; Dodds, S.; Mercer, P.; Smith, P.K.; Walder, J. Inhibition of glycation of albumin and hemoglobin by acetylation in vitro and in vivo. J. Lab. Clin. Med. 1986, 108, 277–285. [Google Scholar] [CrossRef]
- Harding, J.J.; Egerton, M.; Harding, R.S. Protection against cataract by aspirin, paracetamol and ibuprofen. Acta Ophthalmol. 1989, 67, 518–524. [Google Scholar] [CrossRef]
- Roberts, K.A.; Harding, J.J. Ibuprofen, a putative anti-cataract drug, protects the lens against cyanate and galactose. Exp. Eye Res. 1990, 50, 157–164. [Google Scholar] [CrossRef]
- Rasheed, S.; Sánchez, S.S.; Yousuf, S.; Honoré, S.M.; Choudhary, M.I. Drug repurposing: In-vitro anti-glycation properties of 18 common drugs. PLoS ONE 2018, 13, e0190509. [Google Scholar] [CrossRef]
- Booth, A.A.; Khalifah, R.G.; Hudson, B.G. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: Comparison with aminoguanidine. Biochem. Biophys. Res. Commun. 1996, 220, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.A.; Hassan, O.F.; Galal, O.; Mansour, D.F.; El-Khatib, A. Beneficial effects of benfotiamine, a NADPH oxidase inhibitor, in isoproterenol-induced myocardial infarction in rats. PLoS ONE 2020, 15, e0232413. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, S.Y.; Xu, P.; Babcock, S.A.; Dolence, E.K.; Brownlee, M.; Li, J.; Ren, J. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J. Cell Mol. Med. 2009, 13, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.O.; Alderson, N.L.; Chachich, M.E.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: Evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J. Biol. Chem. 2003, 278, 42012–42019. [Google Scholar] [CrossRef]
- Hagiwara, S.; Gohda, T.; Tanimoto, M.; Ito, T.; Murakoshi, M.; Ohara, I.; Yamazaki, T.; Matsumoto, M.; Horikoshi, S.; Funabiki, K.; et al. Effects of pyridoxamine (K-163) on glucose intolerance and obesity in high-fat diet C57BL/6J mice. Metabolism 2009, 58, 934–945. [Google Scholar] [CrossRef]
- Unoki-Kubota, H.; Yamagishi, S.; Takeuchi, M.; Bujo, H.; Saito, Y. Pyridoxamine, an inhibitor of advanced glycation end product (AGE) formation ameliorates insulin resistance in obese, type 2 diabetic mice. Protein Pept. Lett. 2010, 17, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants 2019, 8, 344. [Google Scholar] [CrossRef]
- Maessen, D.E.; Brouwers, O.; Gaens, K.H.; Wouters, K.; Cleutjens, J.P.; Janssen, B.J.; Miyata, T.; Stehouwer, C.D.; Schalkwijk, C.G. Delayed Intervention With Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet-Induced Obese Mice. Diabetes 2016, 65, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibasaki, T.; Park, J.H.; Hidaka, S.; Takahashi, T.; Ono, A.; Song, D.K.; Seino, S. Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes 2015, 64, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, X.; Liu, J.; Sun, X.; Rousselle, T.; Ren, D.; Tong, N.; Li, J. AMPK is associated with the beneficial effects of antidiabetic agents on cardiovascular diseases. Biosci. Rep. 2019, 39, BSR20181995. [Google Scholar] [CrossRef] [PubMed]
- Reyaz, A.; Alam, S.; Chandra, K.; Kohli, S.; Agarwal, S. Methylglyoxal and soluble RAGE in type 2 diabetes mellitus: Association with oxidative stress. J. Diabetes Metab. Disord. 2020, 19, 515–521. [Google Scholar] [CrossRef]
- Semo, D.; Obergassel, J.; Dorenkamp, M.; Hemling, P.; Strutz, J.; Hiden, U.; Müller, N.; Müller, U.A.; Zulfikar, S.A.; Godfrey, R.; et al. The Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitor Empagliflozin Reverses Hyperglycemia-Induced Monocyte and Endothelial Dysfunction Primarily through Glucose Transport-Independent but Redox-Dependent Mechanisms. J. Clin. Med. 2023, 12, 1356. [Google Scholar] [CrossRef]
- Tusa, I.; Menconi, A.; Tubita, A.; Rovida, E. Pathophysiological Impact of the MEK5/ERK5 Pathway in Oxidative Stress. Cells 2023, 12, 1154. [Google Scholar] [CrossRef]
- Niedzielski, M.; Broncel, M.; Gorzelak-Pabiś, P.; Woźniak, E. New possible pharmacological targets for statins and ezetimibe. Biomed. Pharmacother. 2020, 129, 110388. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Pentoxifylline for vascular health: A brief review of the literature. Open Heart 2016, 3, e000365. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Wu, S.; Jin, J.; Li, W.; Wang, N. Calcium dobesilate for diabetic retinopathy: A systematic review and meta-analysis. Sci. China Life Sci. 2015, 58, 101–107. [Google Scholar] [CrossRef]
- Haller, H.; Ji, L.; Stahl, K.; Bertram, A.; Menne, J. Molecular Mechanisms and Treatment Strategies in Diabetic Nephropathy: New Avenues for Calcium Dobesilate-Free Radical Scavenger and Growth Factor Inhibition. Biomed. Res. Int. 2017, 2017, 1909258. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Indurthi, V.S.; Leclerc, E.; Vetter, S.W. Calorimetric investigation of diclofenac drug binding to a panel of moderately glycated serum albumins. Eur. J. Pharm. Sci. 2014, 59, 58–68. [Google Scholar] [CrossRef]
- Raval, A.D.; Thakker, D.; Rangoonwala, A.N.; Gor, D.; Walia, R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst. Rev. 2015, 1, CD009403. [Google Scholar] [CrossRef]
- Borg, D.J.; Forbes, J.M. Targeting advanced glycation with pharmaceutical agents: Where are we now? Glycoconj. J. 2016, 33, 653–670. [Google Scholar] [CrossRef]
- Sambon, M.; Wins, P.; Bettendorff, L. Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine. Int. J. Mol. Sci. 2021, 22, 5418. [Google Scholar] [CrossRef]




| Experimental Model | MGO/MAGEs and Associated Major Findings | Ref./Year |
|---|---|---|
| SD rats treated with 60% Fru (in chow) for 16 weeks | Upon Fru treatment (in comparison with control SD rats): Increase in systolic blood pressure. In blood serum: Increase in MGO; Decrease in GSH. In aorta: Increase in MGO, hydrogen peroxide and CEL; Decrease in eNOS in endothelial cells; No change in GSH. In mesenteric artery: Increase in CEL and CML; Increase in wall thickness and decrease in the vessel lumen. | [31]/2008 |
| Lean, obese, and diabetic Zucker rats | In obese and/or diabetic Zucker rats (as compared to lean Zucker rats control): In the serum: Increase in Glc, MGO, and fructose; Increase in insulin in obese rats, but decrease in insulin in diabetic rats. In aortas: Increase in MGO and fructose; Upregulation of GLUT-5, fructokinase, and aldolase B (at mRNA levels); Increase in aldose reductase and sorbitol in diabetic rats. | [89]/2011 |
| Sprague Dawley (SD) rats treated with 60% fructose (Fru) (in chow) for 16 weeks | Upon Fru treatment (in comparison with control SD rats): In aortas: Increase in MGO and Fru; Upregulation of GLUT-5, fructokinase, and aldolase B (at mRNA levels). | [89]/2011 |
| SD rats treated with Fru-enriched diet (60% fructose, 22% crude proteins, 5% crude fat, 5% crude fiber, 6% ash, and 2% minerals) for 9 weeks | Upon Fru treatment (in comparison with control SD rats): Increase in blood pressure. In blood serum: Increase in MGO, TAG, and insulin; No changes in total cholesterol, HDL-cholesterol, HbA1c, or Glc. In the adipose tissue: Increase in MGO and PI3K protein; Decrease in insulin-induced Glc uptake; Decreased PI3K recruitment to phosphorylated IRS-1; No changes in IR, IRS-1 expression, or phosphorylation. | [94]/2007 |
| SD rats treated with 60% Fru (in chow) for 16 weeks | Upon Fru treatment (in comparison with control SD rats): Increase in blood pressure and vascular remodeling; In blood plasma: Increase in MGO. In aorta: Increase in MGO and Akt1 phosphorylation at Ser-473. | [95]/2011 |
| SD rats treated with MGO (administered using continuous infusion with a minipump for 4 weeks; 60 mg/kg/day) | Upon MGO treatment (in comparison with control rats): In aorta: Increase in Akt1 phosphorylation at Ser-473. | [95]/2011 |
| Wistar rats infused with MGO (75 mg/kg body weight/day for 8 weeks) | Upon MGO treatment (in comparison with control rats): In blood plasma: increase in CML. In heart tissue: Decrease in catalase, SOD, and GSH; Increase in cardiac fibrosis; Upregulated expression of RAGE (3.5 fold), TGF-β (4.4 fold), SMAD2 (3.7 fold), SMAD3 (6.0 fold), IL-6 (4.3 fold), and TNF-α (5.5 fold). | [96]/2017 |
| Wistar rats fed with MGO diluted in the daily water (75 mg/kg/day) for 8 weeks | Upon MGO treatment (in comparison with control rats): In blood plasma/serum: Increase in free fatty acids; No change in glycemia (fasting and 2 h after glucose administration), glycated haemoglobin, insulinemia and total cholesterol, triglycerides, and adiponectin levels. In the adipose tissue: Increase in CEL and fibrosis. In the adipose tissue of MGO-fed Wistar rats after blood supply reduction: Increase in ERK1/2 phosphorylation (p-ERK1 plus p-ERK2); Increase in perilipin A degradation (due to MGO-induced glycation); Decrease in IkBa, PPARγ expression, and Akt activation. | [97]/2013 |
| Wistar rats treated with MGO (administered intraperitoneally over 5 consecutive days each week for 7 weeks: 50 mg/kg for weeks 1 and 2; 60 mg/kg for weeks 3 and 4; 75 mg/kg for weeks 5, 6 and 7) | Upon MGO treatment (in comparison with control rats): In the blood serum: Increase in cholesterol, creatinine, and fructosamine; No change in Glc; In skin vasculature: Upregulation of AGEs and RAGE; Progressive thickening of blood vessel wall followed by its detachment from matrix, luminal occlusion, and endothelial cell death ending up with vessel disappearance; No vasodilation upon nitroglycerine treatment; Proinflammatory and profibrotic response (increased IL-1β, TNF-α, CTGF, and TGF-β; disturbances in wound healing). | [98]/2005 |
| SD rats treated with MGO (1% MGO in tap drinking water) for 4 weeks | Upon MGO treatment (in comparison with control rats): Increase in insulin resistance without increase in blood pressure. In the kidney: Increase in CEL and nitrotyrosine. | [99]/2009 |
| SD rats treated with MGO (17.25 mg/kg in a single intraperitoneal injection, or 6.48/50 mg/kg as intravenous infusion) | Upon MGO treatment (in comparison with control rats): Impairment in Glc tolerance. In the blood plasma: Increase in insulin; Decrease in glutathione. In the visceral adipose tissue: Decrease in insulin-stimulated glucose uptake; Reduced plasma membrane GLUT-4 and IRS-1 tyrosine phosphorylation; No change in insulin receptor and IRS-1 protein expression. | [100]/2010 |
| C57/BL6 mice treated with MGO (administered intraperitoneally over 5 consecutive days each week for 7 weeks: 50 mg/kg for weeks 1 and 2; 60 mg/kg for weeks 3 and 4; and 75 mg/kg for weeks 5, 6, and 7) | Upon MGO treatment (in comparison with control mice): Increase in systemic insulin resistance. In the blood serum: Reduction of insulin-stimulated increase in serum NO. In aortas: Decrease in insulin-induced activation of Akt and eNOS; Induction of ERK ½ phosphorylation and endothelin-1 release (comparable to insulin effect). | [101]/2014 |
| C57/BL6 mice treated with MGO (administered intraperitoneally over 5 consecutive days each week for 7 weeks: 50 mg/kg for weeks 1 and 2; 60 mg/kg for weeks 3, 4, and 5; and 75 mg/kg for weeks 6 and 7) | Upon MGO treatment (in comparison with control mice): In the blood serum/plasma: Decrease in the levels of SOD, CAT, and GPX; Increase in MDA; Increase in proinflammatory cytokines (IL-1β and IL-6) and the anti-inflammatory cytokine IL-10. In aortas: Increase in aorta thickness and apoptosis; Decrease in Nrf2 expression and Akt phosphorylation. | [102]/2022 |
| SD rats treated with MGO (administered using continuous infusion with a minipump for 4 weeks; 60 mg/kg/day) | Upon constant MGO treatment (in comparison with control rats): In the blood plasma: Increase in Glc, total cholesterol, TAG, and free fatty acids; Decrease in fasting insulin, HDL, and GSH. In the pancreas/pancreatic β-cells: Enhanced formation of CML and increased apoptosis; Reduced GLUT-2 (=decreased Glc uptake) and glucokinase; Lowered insulin secretion—downregulation of factors promoting insulin expression (PDX-1 and MafA) and upregulation of the factor inhibiting insulin expression (C/EBPβ); Upregulation of NF-κB and RAGE. In the adipose tissue: Decrease in insulin-stimulated Glc uptake; Reduced plasma membrane GLUT-4, IRS-1 phosphorylation, and PI3K activity; No change in insulin receptor or IRS-1 protein expression. Decrease in GSH in pancreas and skeletal muscle. | [103]/2011 |
| SD rats treated with MGO (administered using continuous infusion with a minipump for 4 weeks; 24 mg/day) | Upon constant MGO treatment (in comparison with control rats): Increase in blood pressure. In blood plasma: Increase in norepinephrine, epinephrine, dopamine, angiotensin, renin, and aldosterone. In aortas: Elevated adrenergic α1D receptor, angiotensin AT1 receptor, and angiotensin protein and mRNA. In the kidney: Increase in angiotensin AT1 receptor, renin, and angiotensin protein and mRNA. In aortas and kidney: Increase in phosphorylated Erk 1/2 (p-Erk 1/2) and NFATc expression. | [104]/2014 |
| Wistar rats treated with MGO (50–75 mg/kg/day, in drinking water for 3 months) | Upon MGO treatment (in comparison with control Wistar rats): In aorta: Decline in NO-dependent vascular relaxation; Increase in superoxide, nitrotyrosine, MCP-1, AGEs, and RAGE. In urine: Increase in 8-OHdG. | [105]/2012 |
| Goto–Kakizaki (GK) rats (spontaneously diabetic—T2DM) treated with MGO (50–75 mg/kg/day, in drinking water for 3 months) | Upon MGO treatment (in comparison with control GK rats): In aorta: Decline in NO-dependent vascular relaxation; Increase in nitrotyrosine and RAGE. In urine: Increase in 8-OHdG. | [105]/2012 |
| Wistar rats treated with MGO (50–75 mg/kg/day, in drinking water for 14 weeks) | Upon MGO treatment (in comparison with control Wistar rats): In blood plasma/serum: Increase in free fatty acids; Decrease in adiponectin. In urine: Increase in 8-OHdG. In the adipose tissue: Increase in MGO, AGEs, glycoconjugates, fibrosis, and TGF-β (but not its cleaved form); Increase in proapoptotic factors (decreased Bcl2/Bax ratio and upregulation of caspase 3); Increase in proinflammatory factors (MCP-1 and F4/80); Decrease in VEGF but unchanged angiopoietin 2. | [106]/2012 |
| Hereditary hypertriglyceridaemic rats (HHTg) treated with MGO (administered intragastrically three times a week at a dose of 0.5 mg/kg for 4 weeks) | Upon MGO treatment (in comparison with control HHTg rats): In blood serum: Increase in non-fasting Glc and insulin; Increase in proinflammatory MCP-1 and TNFα. In white adipose tissue: Decrease in the conversion of Glc into lipids upon insulin stimulation; Increase in adrenaline-stimulated lipolysis; Increase in saturated fatty acids and decrease in polyunsaturated fatty acids; Decrease in Nrf2 expression; Increase in MCP-1 and TNFα expression; No effect on Glo1 or HIF-1 expression. | [107]/2020 |
| Spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY) | In comparison with normal WKY, in SHR rats: Higher MGO level in blood plasma and kidney (increasing with age); Higher CML and CEL staining in the kidney; Decreased GSH and GSH/GSSG ratio in the kidney of the oldest 20-week rats. | [108]/2004 |
| SHR and WKY rats | In comparison with normal WKY, in SHR rats: Higher MGO level in blood plasma and aorta (increasing with age); Higher MGO level in the liver and kidney (but not in the heart) in 13-week rats; Higher CML and CEL staining in the aorta (mostly in endothelial cells, lower in smooth muscle cells); Increased oxidative stress (superoxide anion and hydrogen peroxide) in 13-week rats aortas; Decreased GSH in 13-week rats’ aortas; Decreased activities of glutathione peroxidase and reductase in 13-week rats’ aortas; Increased activity of SSAO in blood plasma; No difference in blood plasma GSH. | [109]/2005 |
| SHR and WKY rats | In comparison with normal WKY, in SHR rats: Higher MGO level in blood plasma and aorta; Higher level of AP and CEL in the aorta and mesenteric artery; Increase in oxidative stress in aortic tissue (enhanced level of superoxide anion and nitric oxide, decreased GSH); Changes in nitric oxide synthases expression in aortic tissue (increase in iNOS and decrease in eNOS); Worsening of morphologic changes and endothelium-dependent relaxation in mesenteric artery. | [110]/2007 |
| SHR and WKY rats | In comparison with normal WKY, in SHR rats: Higher levels of AP and CEL in the mesenteric artery; Increase in oxidative stress in mesenteric artery. | [111]/2012 |
| SHR and WKY rats | In comparison with normal WKY, in SHR rats: In the serum: Similar Glc, increase in MGO, fructose, and insulin; In aortas: Increase in MGO and fructose; Upregulation of GLUT-5, fructokinase, and aldolase B (at mRNA levels). | [89]/2011 |
| Medications (Agents) | Possible Mechanism of Action: Key Points in the MAGE/RAGE or AGE/RAGE Axis, Biochemical and Physiological Effects | Ref. |
|---|---|---|
| 1. Antihyperglycemic agents used in the treatment of type 2 diabetes (blood glucose-lowering agents) | ||
| Biguanides | ||
| Metformin | In vitro: MGO scavenger, inhibits carbonyl stress; reduces ↓ cross-linking, ↓ AGE, and ↓ HbA1c formation; restores antioxidant levels in monocytes (THP-1 cells) and erythrocytes; reduces mitochondrial complex I activity; activates ↑ AMPK. Human studies: dose-dependently reduces plasma ↓ MGO; increases ↑ Glo1 activity in peripheral blood cells and atherosclerotic lesions; scavenges MGO to form imidazolinone metabolite, which is excreted in urine. | [17] [102] [223] [233] [234] [235] [236] [237] |
| Buformin | In vitro: reduces ↓ AGE formation (a more potent inhibitor than metformin) and ↓ cross-linking. | [234] |
| Sulfonylureas | ||
| Glibenclamide (=glyburide) | In vitro and Animal studies: reduces ↓ AGE formation; KATP channel antagonist; reverses the activation of JNK (stress-activated protein kinase) by blocking KATP; reduces endothelial cell dysfunction by inhibiting activation of the JNK/p38 MAPK pathway (study in HAECs from healthy and T2DM donors), and the effect is mediated in part via KATP and protection of glycation sites. | [155] [238] |
| Gliclazide | In vitro and Animal studies: reduces ↓ AGE formation; KATP channel antagonist; induces activation of CaMKKβ (Ca2+/calmodulin-dependent protein kinase kinase β) and AMPK; inhibits vascular smooth muscle cell proliferation through the CaMKKβ-AMPK pathway; effects of KATP on AMPK activity are mediated by the regulation of intracellular Ca2+ levels. | [239] [240] |
| Glipizide | In vitro: reduces ↓ AGE formation; restores antioxidant levels in monocytes (THP-1 cells) and erythrocytes. | [235] |
| Glimepiride | Human studies: increases plasma ↑ esRAGE and decreases ↓ RAGE expression in peripheral mononuclear cells (to a lesser extent than pioglitazone). | [241] |
| Thiazolidinediones | ||
| Pioglitazone | In vitro: reduces ↓ AGE and ↓ HbA1c formation; reduces ↓ RAGE and ↓ RAGE mRNA expression in human endothelial cells, thereby limiting EC susceptibility to proinflammatory AGE effects; suppresses NF-κB levels and alleviates cellular oxidative stress and inflammation; preferentially binds to protein and alleviates protein conformational changes; pioglitazone restores cellular antioxidants and reduces levels of IL-6 and TNF-α by decreasing expression of membrane RAGE and NF-κB; pioglitazone and rosiglitazone inhibit platelet aggregation by activating ↑ AMPK. Human studies: increases in circulating ↑ sRAGE or sRAGE/esRAGE; this effect is not observed with rosiglitazone; pioglitazone suppresses RAGE expression and increases circulating sRAGE/esRAGE (activity is not necessarily dependent on plasma glucose or insulin resistance levels). | [241] [242] [243] [244] [245] [237] |
| Rosiglitazone | ||
| 2. Agents for the treatment of cardiovascular conditions | ||
| Angiotensin II receptor antagonists (blockers, ARBs) and angiotensin-converting enzyme inhibitors | ||
| ARBs Candesartan Irbesartan Losartan Olmesartan Telmisartan Valsartan | In vitro and Animal studies: reduces ↓ AGE (argpyrimidine, pentosidine and CML) formation; chelates transition metal cations, acts as an antioxidant, inhibits ↓ ROS and ↓ RCS formation; the effect on AGE formation is common to all tested ARBs; a similar but milder effect is observed with ACE inhibitors (IC50 of pentosidine formation in BSA-arabinose model: valsartan > candesartan > olmesartan > temocaprilat > enalaprilat > irbesartan = losartan = telmisartan > captopril > perindoprilat); Candesartan attenuates vascular dam age in diabetic retinopathy by restoring Glo1 function and reducing ↓ ●NO; restores both ↑ Glo1 activity and ↑ Glo1 mRNA levels; reduces ↓ mRNA levels of ICAM-1 (intercellular adhesion molecule), VEGF (vascular endothelial growth factor), TNF-α, and iNOS; reduces ↓ total AGEs, MAGEs, and argpyrimidine in retina and plasma; Olmesartan dose-dependently reduces the development of diabetic nephropathy in rats with type 2 diabetes, as evidenced by reductions in proteinuria and pathologic evidence of diabetic glomerulosclerosis. | [207] [208] [246] [247] [248] |
| ACE inhibitors Captopril Enalaprilat (active metabolite of enalapril) Perindoprilat (active metabolite of perindopril) Temocaprilat (active metabolite of temocapril) | ||
| Calcium channel antagonists (blockers) | ||
| With vascular effects Amlodipine Isradipine Lacidipine Nifedipine | In vitro: acts as an antioxidant (lacidipine > semotiadil > amlodipine > nifedipine > diltiazem); inhibits ↓ glycation and ↓ glycoxidation; inhibits the copper-mediated oxidation of non-glycated and glycated LDL. | [249] |
| With direct cardiac effects Diltiazem | ||
| Semotiadil (experimental) | ||
| Hydrazinophthalazine derivatives (arteriolar smooth muscle, agents acting on) | ||
| Hydralazine | In vitro: MGO scavenger; inhibits carbonyl stress; inhibits the formation of AGEs (pentosidine and CML); chelates transition metal cations, acts as an antioxidant, and inhibits the formation of ↓ ROS; inhibits the glycation of LDL and prevents the formation of model foam cells from RCS-modified low-density lipoproteins. Animal studies: the effect of hydralazine (5 mg) is similar to that of olmesartan (1 mg), but reached statistical significance only for renal pentosidine content. | [230] [248] [250] |
| Statins (lipid modifying agents, HMG-CoA reductase inhibitors) | ||
| Atorvastatin | In vitro: atorvastatin o- and p-OH metabolites are potent antioxidants and protect LDL, VLDL, and HDL from oxidation; the inhibitory effects of these metabolites on HDL oxidation are associated with the protection of paraoxonase activity. Animal studies: reduces ↓ AGEs, effect is associated with upregulation of serum and renal ↑ sRAGE levels, although renal esRAGE mRNA expression is not significantly increased. Human studies: reduces serum levels of ↓ AGEs in hypercholesterolemic T2DM patients without CVD, but does not reduce fasting glucose or HbA1c levels; AGE changes do not correlate with lipid parameters; atorvastatin tends to reduce serum levels of 8-OHdG (8-hydroxy-2-deoxyguanosine), but not significantly. | [224] [251] [252] |
| Lovastatin | In vitro: increases the levels of ↑ sRAGE by enhancing the shedding of full-length RAGE, but does not affect the secretion of esRAGE. | [218] |
| Cerivastatin | Human studies: reduces levels of ↓ CML-derived AGEs (compared to the placebo group); effect on CML-AGEs correlates with reduction in LDL cholesterol and LDL apolipoprotein B; HbA1c is not changed. | [225] |
| Fluvastatin | In vitro: inhibits mitogen-activated protein kinase kinase ↓ MEK (MAPK/ERK kinase, also known as MAP2K, MAPKK), which downregulates EGR-1 (early growth response protein 1) transcription and leads to decreased levels of CTGF (connective tissue growth factor), and consequently reduces proliferation, migration, and ECM (extracellular matrix) accumulation in AGE-induced human aortic smooth muscle cells (VSMCs); activates ↑ PPAR-γ in HASMCs, but not in HUVECs; induces COX-2 expression in HASMCs, but not in HUVECs; suppresses migration and proliferation of HASMCs and inhibits lipopolysaccharide-induced expression of MCP-1 (monocyte chemoattractant protein-1) and TNF-α in HASMCs. Animal studies: inhibits atherosclerotic lesion formation in Apoe−/− mice; increases transcriptional activity of ↑ PPAR-γ; and decreases aortic expression of ↓ MCP-1 and ↓ TNF-α. | [253] [254] [255] |
| Pitavastatin | ||
| Pravastatin | In vitro: inhibits ↓ AGE-induced upregulation of RAGE mRNA levels; reduces ROS generation and apoptosis in human renal proximal tubular cells. | [256] |
| Rosuvastatin | ||
| Simvastatin | In vitro: reduces ↓ AGE-induced oxidative stress (ROS overproduction) in endothelial cells; diminishes neutrophil adhesion to endothelium; reduces ↓ RAGE mRNA expression, and non-statistically increases ↑ PPAR-γ mRNA expression (PPAR-γ has a protective effect on ECs by inhibiting endothelin-1 release and attenuating/preventing the endothelial inflammatory response). Animal studies: 12-week treatment attenuates AGE-induced proliferation of aortic smooth muscle cells in Sprague Dawley rats and reduces ↓ NF-κβ and ↓ MAPK activation in these cells. | [213] [257] |
| Peripheral vasodilators (purine derivatives) | ||
| Pentoxifylline | In vitro: reduces ↓ AGE and ↓ HbA1c formation | [237] |
| Vasoprotectives (e.g., for the treatment of peripheral vascular disease) | ||
| Antivaricose agents Calcium dobesylate | In vitro: reduces ↓ AGE formation; ROS scavenger; inhibits ↓ ROS formation, acts as an antioxidant; protects glycation reaction substrates from ROS and MGO-induced modifications; reduces impairment of sarcoplasmic reticulum calcium handling and ↓ ROS formation in rat cardiomyocytes caused by high glucose and high lipid levels. Human studies and Meta-analyses: reduces blood-retinal barrier permeability as measured by the posterior vitreous penetration ratio (PVPR); effect is manifest regardless of the degree of metabolic control and the use of antihypertensive and lipid-lowering agents; has a significant beneficial effect on the control of hemorrhages and the overall development of diabetic retinopathy. | [231] [258] [259] [260] [261] [262] |
| Bioflavonoids (capillary stabilizing agents) Diosmin (diosmetin-7-O-rutinoside) Hesperidin (hesperetin-7-O-rutinoside) Rutin (quercetin-3-O-rutinoside) Troxerutin (trihydroxyethylrutin) Isoquercitrin (quercetin-3-O-glucoside) | In vitro: MGO scavenger (not diosmin or troxerutin), ROS scavenger, inhibits the formation of ↓ ROS and ↓ RCS, acts as an antioxidant, chelates transition metal cations; reduces ↓ AGE formation; Human studies: quercetin-3-O-glucoside and hesperidin reduce plasma ↓ MGO levels (by ~10–11%) in (pre)hypertensive and healthy subjects; but Glo1 expression is not significantly altered; The combination of hesperidin and trans-resveratrol (tRES-HESP) induces ↑ Glo1 expression and counteracts MGO accumulation in overweight and obese subjects; reverses insulin resistance, improves dysglycemia and low-grade inflammation; MGO metabolic variables correlate with BMI, dysglycemia, vascular inflammation, blood pressure, and dyslipidemia. Epidemiologic studies and Meta-analyses: the increase in total flavonoid intake is linearly associated with a lower risk of cardiovascular disease; dose-response analysis showed that consumption of 200 mg/day of total flavonoids was associated with the lowest risk of all-cause mortality. | [210] [212] [228] [229] [231] [263] [264] [265] [266] [267] [268] [269] |
| 3. Anti-inflammatory, analgesic and antipyretic agents (including nonsteroidal anti-inflammatory drugs NSAIDs) | ||
| Acetylsalicylic acid (=aspirin) | In vitro: reduces ↓ glycation of albumin and hemoglobin (not salicylic acid), blocks at least one of the major glycation sites of HSA. Animal studies: reduces glycohemoglobin and glycoalbumin levels in diabetic rats. Human studies: low doses protect against cataracts. | [270] [271] [272] |
| Diclofenac | In vitro: reduces ↓ glycation of albumin and hemoglobin, blocks at least one of the major glycation sites of HSA. | [270] |
| Ibuprofen | In vitro: prevents modification of lens proteins by carbonylation and nonenzymatic glycation; reduces cyanate and galactose binding but not glucose-6-phosphate binding; protects against opacities; appears to have a different mechanism of action than aspirin. Human studies: low doses protect against cataracts. | [272] [273] |
| Nimesulide Mefenamic acid Meloxicam Piroxicam | In vitro: reduces ↓ AGE formation; acts as an antioxidant, chelates transition metal cations. | [274] |
| Paracetamol | Human studies: low doses protect against cataracts. | [272] |
| 4. Selected B vitamins | ||
| Thiamine pyrophosphate (B1) | In vitro: reduces ↓ AGE formation (thiamine and thiamine monophosphate are not inhibitors); is essential for maintaining cellular defenses against oxidative stress. | [275] |
| Benfotiamine (a lipid soluble thiamine derivative) | Animal studies: reduces ↓ AGE formation; activates antioxidant defense mechanisms, a ↓ NADPH oxidase inhibitor (this enzyme plays an essential role in ROS production and myocardial cytotoxicity); improves markers of oxidative stress, inflammation, and apoptosis; inhibits ↓ NF-κB by activating transketolase in diabetic animals, prevents experimental diabetic retinopathy; attenuates or abolishes diabetes-induced increase in cardiac levels of ↓ MGO, ↓ AGEs (MAGEs), ↓ RAGE, and ↓ cross-linked collagen without affecting hypertriglyceridemia and hypercholesterolemia. Human studies: reduces ↓ CML-derived AGE levels. | [226] [276] [277] |
| Pyridoxamine, pyridoxal, pyridoxal phosphate, pyridoxine (B6) | In vitro: GO and MDA scavenger, reduces ↓ AGE formation; reduces ↓ ALE formation (but pyridoxine is slightly effective at the highest concentrations). Animal studies: inhibits the ↓ AGE/RAGE pathway; increases ↑ Glo1 expression in visceral and perivascular adipose tissue; pyridoxamine reduces MGO-induced atherosclerosis and inflammation; improves glucose tolerance and insulin metabolism in obese mice; prevents adipose tissue inflammation and vascular dysfunction; reduces fasting insulin levels and improves insulin sensitivity in obese and type 2 diabetic mice, most likely by scavenging MGO and inhibiting AGE formation. Human studies: reduces ↓ MGO (9%), ↓ MAGEs (MG-H1), ↓ sVCAM-1 (soluble vascular cell adhesion molecule-1), and ↓ sICAM-1 (soluble intercellular adhesion molecule-1), but does not affect insulin sensitivity or vascular function in abdominally obese subjects; reduction of adhesion markers is promising in the pathogenesis of endothelial damage and atherosclerosis. | [209] [220] [227] [230] [232] [275] [278] [279] [280] [281] [282] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdowska, I.; Matusiewicz, M.; Fecka, I. Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies. Molecules 2023, 28, 7742. https://doi.org/10.3390/molecules28237742
Berdowska I, Matusiewicz M, Fecka I. Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies. Molecules. 2023; 28(23):7742. https://doi.org/10.3390/molecules28237742
Chicago/Turabian StyleBerdowska, Izabela, Małgorzata Matusiewicz, and Izabela Fecka. 2023. "Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies" Molecules 28, no. 23: 7742. https://doi.org/10.3390/molecules28237742
APA StyleBerdowska, I., Matusiewicz, M., & Fecka, I. (2023). Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies. Molecules, 28(23), 7742. https://doi.org/10.3390/molecules28237742