Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. SEM–EDS Analysis
2.3. FT-IR Analysis
2.4. XPS Analysis
2.5. TEM Analysis
2.6. DR/UV-Vis Analysis Analysis
2.7. Photocatalytic Activities
2.8. Optimization of the Experimental Conditions for 4-NP Photodegradation
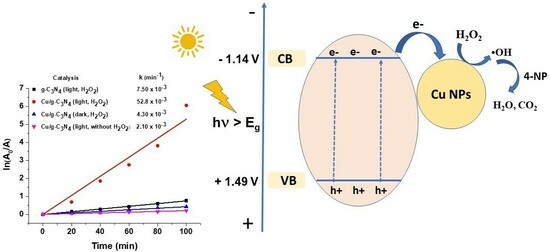
2.8.1. Effect of Copper Modification and the Presence of H2O2
2.8.2. Effect of Doped Copper Content
2.8.3. Effect of the Photocatalyst Concentration
2.8.4. Effect of the Initial Solution pH
2.8.5. Effect of the 4-NP Initial Concentration
2.9. Photodegradation of 4-NP, 2,4-di-NP and 2,4,6-tri-NP
2.10. Reusability of the Photocatalyst
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Preparation of g-C3N4 Catalyst
3.2.2. Preparation of Cu/g-C3N4 Catalysts
3.3. Sample Characterization
3.4. Photocatalytic Degradation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent Progress on Semiconductor Heterogeneous Photocatalysts in Clean Energy Production and Environmental Remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Li, Q.; Hou, X.; Wang, L.-N.; Huang, Z.-H. Facile fabrication of three-dimensional interconnected nanoporous N-TiO2 for efficient photoelectrochemical water splitting. J. Mater. Sci. Technol. 2018, 34, 955–960. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2021, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Bharagav, U.; Ramesh Reddy, N.; Nava Koteswara Rao, V.; Ravi, P.; Sathish, M.; Rangappa, D.; Prathap, K.; Shilpa Chakra, C.; Shankar, M.V.; Appels, L.; et al. Bifunctional g-C3N4/carbon nanotubes/WO3 ternary nanohybrids for photocatalytic energy and environmental applications. Chemosphere 2023, 311, 137030. [Google Scholar] [CrossRef] [PubMed]
- Manchala, S.; Gandamalla, A.; Rao, V.N.; Venkatakrishnan, S.M.; Shanker, V. Solar-light responsive efficient H2 evolution using a novel ternary hierarchical SrTiO3/CdS/carbon nanospheres photocatalytic system. J. Nanostructure Chem. 2022, 12, 179–191. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Li, Z.; Wang, Q. Renewable biomass-derived carbon-supported g-C3N4 doped with Ag for enhanced photocatalytic reduction of CO2. J. Colloid Interface Sci. 2022, 606, 1311–1321. [Google Scholar] [CrossRef]
- Kumar, A.; Kashyap, S.; Sharma, M.; Krishnan, V. Tuning the surface and optical properties of graphitic carbon nitride by incorporation of alkali metals (Na, K, Cs and Rb): Effect on photocatalytic removal of organic pollutants. Chemosphere 2022, 287, 131988. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, F.; Cao, F.; Zhao, X.; Meng, S.; Cui, Y. Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis. Appl. Catal. B Environ. 2017, 206, 417–425. [Google Scholar] [CrossRef]
- Guo, S.; Tang, Y.; Xie, Y.; Tian, C.; Feng, Q.; Zhou, W.; Jiang, B. P-doped tubular g-C3N4 with surface carbon defects: Universal synthesis and enhanced visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Yang, Z.; Shen, R.; Li, H.; Luo, X.; Chen, X.; Li, X. Fabricating the Robust g-C3N4 Nanosheets/Carbons/NiS Multiple Heterojunctions for Enhanced Photocatalytic H2 Generation: An Insight into the Trifunctional Roles of Nanocarbons. ACS Sustain. Chem. Eng. 2017, 5, 2224–2236. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Liu, R.; Bie, Y.; Qiao, Y.; Liu, T.; Song, Y. Design of g-C3N4/TiO2 nanotubes heterojunction for enhanced organic pollutants degradation in waste water. Mater. Lett. 2019, 251, 126–130. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Han, Y.; Ye, H.; Liu, R.; Lan, Y.; Xue, M.; Xie, X.; Yu, S.; Zhang, L.; et al. g-C3N4-based photocatalysts for organic pollutant removal: A critical review. Carbon Res. 2023, 2, 14. [Google Scholar] [CrossRef]
- Gu, J.; Guo, R.; Miao, Y.; Liu, Y.; Wu, G.; Duan, C.; Pan, W. Construction of full spectrum-driven CsxWO3/g-C3N4 heterojunction catalyst for efficient photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 540, 148316. [Google Scholar] [CrossRef]
- Tian, B.; Wu, Y.; Lu, G. Metal-free plasmonic boron phosphide/graphitic carbon nitride with core-shell structure photocatalysts for overall water splitting. Appl. Catal. B Environ. 2021, 280, 119410. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Xiao, K.; Sun, H.; Chan, H.S.; Tsang, T.H.; Yang, S.; Wong, P.K. Photo-assisted separation of noble-metal-free oxidation and reduction cocatalysts for graphitic carbon nitride nanosheets with efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 280, 119456. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-mediated copper doped porous g-C3N4 for efficient photodegradation of antibiotic contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef]
- Li, F.; Zhu, P.; Wang, S.; Xu, X.; Zhou, Z.; Wu, C. One-pot construction of Cu and O co-doped porous g-C3N4 with enhanced photocatalytic performance towards the degradation of levofloxacin. RSC Adv. 2019, 9, 20633–20642. [Google Scholar] [CrossRef]
- Dai, Y.; Gu, Y.; Bu, Y. Modulation of the photocatalytic performance of g-C3N4 by two-sites co-doping using variable valence metal. Appl. Surf. Sci. 2020, 500, 144036. [Google Scholar] [CrossRef]
- Li, X.; Gan, X. Photo-Fenton degradation of multiple pharmaceuticals at low concentrations via Cu-doped-graphitic carbon nitride (g-C3N4) under simulated solar irradiation at a wide pH range. J. Environ. Chem. Eng. 2022, 10, 108290. [Google Scholar] [CrossRef]
- Vasu, D.; Keyan, A.K.; Sakthinathan, S.; Yu, C.-L.; You, Y.-F.; Chiu, T.-W.; Fan, L.; Chen, P.-C. Visible-Light-Active Vanadium and Copper Co-Doped gCN Nanosheets with Double Direct Z-Scheme Heterojunctions for Photocatalytic Removal of Monocrotophos Pesticide in Water. Catalysts 2022, 12, 1489. [Google Scholar] [CrossRef]
- Sakthivel, T.; Ramachandran, R.; Kirubakaran, K. Photocatalytic properties of copper-two dimensional graphitic carbon nitride hybrid film synthesized by pyrolysis method. J. Environ. Chem. Eng. 2018, 6, 2636–2642. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Liyanaarachchi, C.; Samarakoon, U. Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arab. J. Chem. 2023, 16, 104749. [Google Scholar] [CrossRef]
- Le, S.; Jiang, T.; Zhao, Q.; Liu, X.; Li, Y.; Fang, B.; Gong, M. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv. 2016, 6, 38811–38819. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Nitrophenols; ATSDR: Atlanta, GA, USA, 2023. [Google Scholar]
- Majewska, M.; Khan, F.; Pieta, I.S.; Wróblewska, A.; Szmigielski, R.; Pieta, P. Toxicity of selected airborne nitrophenols on eukaryotic cell membrane models. Chemosphere 2021, 266, 128996. [Google Scholar] [CrossRef] [PubMed]
- Larous, S.; Meniai, A.-H. Elimination of organic pollutants from wastewater. Application to p-nitrophenol. Desalin. Water Treat. 2013, 51, 5014–5020. [Google Scholar] [CrossRef]
- Dogan, M.; Temel, F.; Tabakci, M. High-Performance Adsorption of 4-Nitrophenol onto Calix[6]arene-Tethered Silica from Aqueous Solutions. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4191–4202. [Google Scholar] [CrossRef]
- Chaudhary, M.; Suhas; Kushwaha, S.; Chaudhary, S.; Tyagi, I.; Dehghani, M.H.; Stephen Inbaraj, B.; Goscianska, J.; Sharma, M. Studies on the Removal of Phenol and Nitrophenols from Water by Activated Carbon Developed from Demineralized Kraft Lignin. Agronomy 2022, 12, 2564. [Google Scholar] [CrossRef]
- Ramacharyulu, P.V.R.K.; Abbas, S.J.; Sahoo, S.R.; Ke, S.-C. Mechanistic insights into 4-nitrophenol degradation and benzyl alcohol oxidation pathways over MgO/g-C3N4 model catalyst systems. Catal. Sci. Technol. 2018, 8, 2825–2834. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Vinodkumar, T.; Selvaraj, M.; Suryakala, D.; Subrahmanyam, C. Visible light-induced catalytic abatement of 4-nitrophenol and Rhodamine B using ZnO/g-C3N4 catalyst. J. Chem. Sci. 2021, 133, 41. [Google Scholar] [CrossRef]
- Nithya, R.; Ayyappan, S. Novel exfoliated graphitic-C3N4 hybridised ZnBi2O4 (g-C3N4/ZnBi2O4) nanorods for catalytic reduction of 4-Nitrophenol and its antibacterial activity. J. Photochem. Photobiol. A Chem. 2020, 398, 112591. [Google Scholar] [CrossRef]
- Verma, A.; Jaihindh, D.P.; Fu, Y.-P. Photocatalytic 4-nitrophenol degradation and oxygen evolution reaction in CuO/g-C3N4 composites prepared by deep eutectic solvent-assisted chlorine doping. Dalt. Trans. 2019, 48, 8594–8610. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jang, J.; Pawar, R.C.; Ahn, S.-H.; Lee, C.S. Low temperature fabrication of Fe2O3 nanorod film coated with ultra-thin g-C3N4 for a direct z-scheme exerting photocatalytic activities. RSC Adv. 2018, 8, 33600–33613. [Google Scholar] [CrossRef] [PubMed]
- Abazari, R.; Mahjoub, A.R.; Salehi, G. Preparation of amine functionalized g-C3N4@H/SMOF NCs with visible light photocatalytic characteristic for 4-nitrophenol degradation from aqueous solution. J. Hazard. Mater. 2019, 365, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; García-López, E.I.; Marcì, G.; Farrokhnia, A.; Shoushtari, M.Z. Photocatalytic degradation of 4-Nitrophenol by g-C3N4-MCy: Mechanism study and kinetic modeling. J. Photochem. Photobiol. A Chem. 2021, 407, 113004. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171. [Google Scholar] [CrossRef]
- Sharma, P.; Sasson, Y. A photoactive catalyst Ru–g-C3N4 for hydrogen transfer reaction of aldehydes and ketones. Green Chem. 2017, 19, 844–852. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Lin, S.; Chu, W.; Sun, D.; Su, Q.; Ma, S.; Du, G.; Xu, B. Single ruthenium atom supported on g-C3N4 as an efficient photocatalyst for nitrogen fixation in ultra-pure water. Catal. Commun. 2021, 153, 106294. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Wang, Z.; Ho, W.-K. Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4 nanosheets via thermal exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Yang, L.; Ren, X.; Zhang, Y.; Chen, Z.; Wan, J. One-step synthesis of a heterogeneous catalyst: Cu+-decorated triazine-based g-C3N4 nanosheet formation and catalytic mechanism. J. Environ. Chem. Eng. 2021, 9, 105558. [Google Scholar] [CrossRef]
- Pattnaik, S.; Rai, V.K. Insight into the spectroscopic and thermometric properties of titanate phosphors via a novel co-excited laser system. Mater. Sci. Eng. B 2021, 272, 115318. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Li, C.; Gao, M.; Cao, N.; Zhao, X.; Li, W.; Ding, X.; Li, Z.; Du, X.; et al. Facile fabrication metal Cu-decorated g-C3N4 photocatalyst with Schottky barrier for efficient pollutant elimination. Diam. Relat. Mater. 2022, 126, 109116. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Makó, É.; Jakab, M.; Zsirka, B. Coumarin-based quantification of hydroxyl radicals and other reactive species generated on excited nitrogen-doped TiO2. J. Photochem. Photobiol. A Chem. 2021, 404, 112913. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Xuan, T.N.; Thi, D.N.; Ngoc, T.N.; Quoc, K.D.; Németh, M.; Mukhtar, S.; Horváth, O. Effect of Ruthenium Modification of g-C3N4 in the Visible-Light-Driven Photocatalytic Reduction of Cr(VI). Catalysts 2023, 13, 964. [Google Scholar] [CrossRef]
- Cao, J.; Han, F.; Wang, L.; Huang, X.; Cao, Y.; He, P.; Yang, H.; Chen, J.; Li, H. Ru/g-C3N4 as an efficient catalyst for selective hydrogenation of aromatic diamines to alicyclic diamines. RSC Adv. 2020, 10, 16515–16525. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]

















| Catalyst | Eg (eV) | EVB (eV) | ECB (eV) |
|---|---|---|---|
| g-C3N4 | 2.63 | 1.49 | −1.14 |
| Cu/g-C3N4 | 2.56 | 1.45 | −1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen Xuan, T.; Nguyen Thi, D.; Tran Thuong, Q.; Nguyen Ngoc, T.; Dang Quoc, K.; Molnár, Z.; Mukhtar, S.; Szabó-Bárdos, E.; Horváth, O. Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols. Molecules 2023, 28, 7810. https://doi.org/10.3390/molecules28237810
Nguyen Xuan T, Nguyen Thi D, Tran Thuong Q, Nguyen Ngoc T, Dang Quoc K, Molnár Z, Mukhtar S, Szabó-Bárdos E, Horváth O. Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols. Molecules. 2023; 28(23):7810. https://doi.org/10.3390/molecules28237810
Chicago/Turabian StyleNguyen Xuan, Truong, Dien Nguyen Thi, Quang Tran Thuong, Tue Nguyen Ngoc, Khanh Dang Quoc, Zsombor Molnár, Shoaib Mukhtar, Erzsébet Szabó-Bárdos, and Ottó Horváth. 2023. "Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols" Molecules 28, no. 23: 7810. https://doi.org/10.3390/molecules28237810
APA StyleNguyen Xuan, T., Nguyen Thi, D., Tran Thuong, Q., Nguyen Ngoc, T., Dang Quoc, K., Molnár, Z., Mukhtar, S., Szabó-Bárdos, E., & Horváth, O. (2023). Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols. Molecules, 28(23), 7810. https://doi.org/10.3390/molecules28237810