Chiral Separation of Cannabichromene, Cannabicyclol, and Their Acidic Analogs on Polysaccharide Chiral Stationary Phases
Abstract
:1. Introduction
2. Results
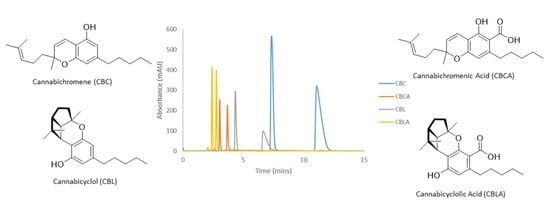
2.1. Analytical Method Development Screening and Optimization
2.2. Preparative Method Development Optimization and Productivity Determination
3. Discussion
3.1. Cannabichromene Preparative Chiral Resolution
3.2. Cannabichromenic Acid Preparative Chiral Resolution
3.3. Cannabicyclol Preparative Chiral Resolution
3.4. Cannabicyclolic Acid Preparative Chiral Resolution
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, J.H.; Scialli, A.R. Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landoni, M.F.; Soraci, A. Pharmacology of chiral compounds: 2-Arylpropionic acid derivatives. Curr. Drug Metab. 2001, 2, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ariens, E.J. Stereoselectivity of bioactive agents: General aspects. In Stereochemistry and Biological Activity of Drugs; Ariens, E.J., Soudijn, W., Timmermans, P.B.M.W.M., Eds.; Blackwell Scientific: Oxford, UK, 1983; pp. 11–33. [Google Scholar]
- Davies, N.M.; Teng, X.V. Importance of chirality in drug therapy and pharmacy practice. Implication for psychiatry. Adv. Pharm. 2003, 1, 242–252. [Google Scholar]
- Patocka, J.; Dvorak, A. Biomedical aspects of chiral molecules. J. Appl. Med. 2004, 2, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Jamali, F.; Mehvar, R.; Pasutto, F.M. Enantioselective aspects of drug action and disposition: Therapeutic pitfalls. J. Pharma. Sci. 1989, 78, 695–715. [Google Scholar] [CrossRef]
- Powell, J.R.; Ambre, J.J.; Ruo, T.I. The efficacy and toxicity of drug stereoisomers. In Drug Stereochemistry. Analytical Methods and Pharmacology, 1st ed.; Wainer, I.W., Ed.; Marcel Dekker Publisher: New York, NY, USA, 1988; pp. 245–270. [Google Scholar]
- Scott, A.K. Stereoisomers and drug toxicity. The value of single stereoisomer therapy. Drug Saf. 1993, 8, 149–159. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Pourseyed Lazarjani, M.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for Quantification of Cannabinoids: A Narrative. Rev. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef]
- National Institutes of Health—National Center for Complementary and Integrative Health (Published November 2019). Available online: https://www.nccih.nih.gov/health/cannabis-marijuana-and-cannabinoids-what-you-need-to-know (accessed on 28 October 2022).
- Mazzoccanti, G.; Ismail, O.H.; D’Acquarica, I.; Villani, C.; Manzo, C.; Wilcox, M.; Cavazzini, A.; Gasparrini, F. Cannabis through the looking glass: Chemo- and enantio-selective separation of phytocannabinoids by enantioselective ultra high performance supercritical fluid chromatography. Chem. Commun. 2017, 53, 12262–12265. [Google Scholar] [CrossRef] [Green Version]
- Tolomeo, F.; Russo, F.; Kaczorova, D.; Vandelli, M.A.; Biagini, G.; Laganà, A.; Capriotti, A.L.; Paris, R.; Fulvio, F.; Carbone, L.; et al. Cis Δ9-tetrahydrocannabinolic acid occurrence in Cannabis sativa L. J. Pharm. Biomed. Anal. 2022, 219, 114958. [Google Scholar] [CrossRef]
- Schafroth, M.A.; Mazzoccanti, G.; Reynoso-Moreno, I.; Erni, R.; Pollastro, F.; Caprioglio, D.; Botta, B.; Allegrone, G.; Grassi, G.; Chicca, A.; et al. Δ9-cis-tetrahydrocannabinol: Natural Occurrence, Chirality, and Pharmacology. J. Nat. Prod. 2021, 84, 2502–2510. [Google Scholar] [CrossRef]
- Zivovinovic, S.; Alder, R.; Allenspach, M.D.; Steuer, C. Determination of cannabinoids in Cannabis sativa L. samples for recreational, medical, and forensic purposes by reversed-phase liquid chromatography-ultraviolet detection. J. Anal. Sci. Tech. 2018, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Aubin, A.J.; Layton, C.; Helmueller, S. Separation of 16 Cannabinoids in Cannabis Flowers and Extracts using Reversed-Phase Isocratic HPLC Method. In Waters Corporation Application Note; Waters Corporation: Milford, MA, USA, 2018. [Google Scholar]
- Mandrioli, M.; Tura, M.; Scotti, S.; Gallina Toschi, T. Fast detection of 10 cannabinoids by RP-HPLC-UV method in Cannabis sativa L. Molecules 2019, 24, 2113. [Google Scholar] [CrossRef] [Green Version]
- Saingam, W.; Sakunpak, A. Development and validation of reverse phase high performance liquid chromatography method for the determination of delta-9-tetrahydrocannabinol and cannabidiol in oromucosal spray from cannabis extract. Rev. Bras. De Farmacogn. 2018, 28, 669–672. [Google Scholar] [CrossRef]
- Patel, B.; Wene, D.; Fan, Z. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharma. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef]
- Levin, S.; Abu-Lafi, S.; Zahalka, J.; Mechoulam, R. Resolution of chiral cannabinoids on amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase: Effects of structural features and mobile phase additive. J. Chroma. A. 1993, 654, 53–64. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Sterin, M.; Levin, S. Role of hydroxyl groups in chiral recognition of cannabinoids by carbamated amylose. J. Chroma. A 1994, 679, 47–58. [Google Scholar] [CrossRef]
- Levin, S.; Sterin, S.; Abu-Lafi, S. Structural features affecting chiral resolution of cannabimimetic enantiomers by amylose 3,5-dimethylphenylcarbamate chiral stationary phase. Chirality 1995, 7, 140–146. [Google Scholar] [CrossRef]
- Yan, G.; Yin, D.; Khanolkar, A.D.; Compton, D.R.; Martin, B.R.; Makriyannis, A. Synthesis and pharmacological properties of 11-hydroxy-3-(1′,1′-dimethylheptyl) hexahydrocannabinol: A high affinity cannabinoid agonist. J. Med. Chem. 1994, 37, 2619–2622. [Google Scholar] [CrossRef]
- Thakur, G.A.; Palmer, S.L.; Harrington, P.E.; Stergiades, I.A.; Tius, M.A.; Makriyannis, M. Enantiomeric resolution of a novel chiral cannabinoid receptor ligand. J. Biochem. Biophys. Meth. 2002, 54, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Tarbox, T.; Dilek, I.; Sreenivasen, U.; Yaser, K. A Validated Chiral HPLC Method for Resolution of Δ8 and Δ9—Tetrahydrocannabinol Enantiomers. Tarbox, T., Ed.; Poster Presented at EAS, Cerilliant Corporation: Round Rock, TX, USA, 2009. [Google Scholar]
- Chiral Technologies. Separation of the Enantiomers of (+/−) Δ8-THC and (+/−) Δ9-THC; Chiral Technologies Application Note; Chiral Technologies: West Chester, PA, USA, 2018. [Google Scholar]
- YMC Europe GmbH. Fast and Easy Achiral & Chiral Analysis of Cannabinoids; YMC Europe GmbH Application Note; YMC Europe GmbH: Dinslaken, Germany, 2018. [Google Scholar]
- Onishi, T.; Umstead, W.J. The separation of cannabinoids on Sub-2 μm immobilized polysaccharide chiral stationary phases. Pharmaceuticals 2021, 14, 1250. [Google Scholar] [CrossRef] [PubMed]
- Felletti, S.; De Luca, C.; Buratti, A.; Bozza, D.; Cerrato, A.; Capriotti, A.L.; Laganà, A.; Cavazzini, A.; Catani, M. Potency Testing of Cannabinoids by Liquid and Supercritical Fluid Chromatography: Where We Are, What We Need. J. Chroma. A. 2021, 1651, 462304. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Buratti, A.; Umstead, W.; Franco, P.; Cavazzini, A.; Felletti, S.; Catani, M. Investigation of retention behavior of natural cannabinoids on differently substituted polysaccharide-based chiral stationary phases under reversed-phase liquid chromatographic conditions. J. Chroma. A. 2022, 1672, 463076–463084. [Google Scholar] [CrossRef] [PubMed]
- Umstead, W.J. The chiral separation of the (+) and (−) enantiomers of cannabidiol (CBD). Cannabis Sci. Technol. 2022, 5, 30–37. [Google Scholar]
- Gutman, A.L.; Etinger, M.; Fedotev, I.; Khanolkar, R.; Nisnevich, G.A.; Pertsikov, B.; Rukhman, I.; Tishin, B. Methods for Purifying trans-(−)-Δ9-Tetrahydrocannabinol and Trans-(+)-Δ9-Tetrahydrocannabinol. US Patent 9,278,083 B2, 8 March 2016. [Google Scholar]
- Toyo’oka, T.; Kikura-Hanajiri, R. A reliable method for the separation and detection of synthetic cannabinoids by supercritical fluid chromatography with mass spectrometry, and its application to plant products. Chem. Pharma. Bull. 2015, 63, 762–769. [Google Scholar] [CrossRef] [Green Version]
- Runco, J.; Aubin, A.; Layton, C. The Separation of Δ8-THC, Δ9-THC, and their Enantiomers by UPC2 using Trefoil Chiral Columns; Waters Corporation Application Note; Waters Corporation: Milford, MA, USA, 2016. [Google Scholar]
- Breitenbach, S.; Rowe, W.F.; McCord, B.; Lurie, I.S. Assessment of ultra high performance supercritical fluid chromatography as a separation technique for the analysis of seized drugs: Applicability to synthetic cannabinoids. J. Chroma. A. 2016, 1440, 201–211. [Google Scholar] [CrossRef]
- Denicola, C.; Barendt, J. StreamLined Cannabinoid and Potency Assays Utilizing HPLC and SFC. In Cannabis Sciences Conference Poster; Chiral Technologies: West Chester, PA, USA, 2018. [Google Scholar]
- Denicola, C.; Barendt, J. Cannabinoid Isolation Models Utilizing Immobilized Chiral Stationary Phases and SFC. In Emerald Conference Poster; Chiral Technologies: West Chester, PA, USA, 2018. [Google Scholar]
- Umstead, W.J. Polysaccharide Chiral Stationary Phases for the Achiral and Chiral Separation of Cannabinoids. In Cannabinoids-Recent Perspectives and Applications in Human Health; James, S.P., Ed.; IntechOpen: London, UK, 2022; pp. 1–18. [Google Scholar]
- DeLong, G.T.; Wolf, C.; Poklis, A.; Lichtman, A.H. Pharmacological evaluation of the neutral constituent of Cannabis sativa, cannabichromene and its modulation by delta-9-tetrahidorcannabinol. Drug Alcohol. Depend. 2010, 112, 126–133. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.; Allara, M.; Bisogno, T.; Petrosino, S.; Stott, C.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Parmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef] [Green Version]
- What Is Cannabicyclolic Acid. Available online: https://leafwell.com/blog/cannabicyclolic-acid/ (accessed on 28 October 2022).
- Development of New Stereoisomeric Drugs (Published May 1992). Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-new-stereoisomeric-drugs (accessed on 28 October 2022).
- Umstead, W.J. The separation of several minor cannabinoids via chiral HPLC. Cannabis Sci. Technol. 2021, 4, 44–51. [Google Scholar]
- De Luca, C.; Buratti, A.; Krauke, Y.; Stephen, S.; Monks, K.; Brighenti, V.; Pellati, F.; Cavazzini, A.; Catani, M.; Felletti, S. Investigating the effect of polarity of stationary and mobile phases on retention of cannabinoids in normal phase liquid chromatography. Anal. Bioanal. Chem. 2022, 414, 5385–5395. [Google Scholar] [CrossRef]
- Berthod, A. (Ed.) Chiral Recognition in Separation Methods; Springer-Verlag: Berlin, Germany, 2010. [Google Scholar]
- Lee, J.; Lee, J.T.; Watts, W.L.; Barendt, J.; Yan, T.Q.; Huang, Y.; Riley, F.; Hardink, M.; Bradow, J.; Franco, P. On the method development of immobilized polysaccharide chiral stationary phases in supercritical fluid chromatography using an extended range of modifiers. J. Chroma. A 2014, 1374, 238–246. [Google Scholar] [CrossRef]
- Marchetti, N.; Cavazzini, A.; Pasti, L.; Dondi, F. Determination of adsorption isotherms by means of HPLC: Adsorption mechanism elucidation and separation optimization. J. Sep. Sci. 2009, 32, 727–741. [Google Scholar] [CrossRef]
- Dose, E.V.; Jacobson, S.; Guiochon, G. Determination of isotherms from chromatographic peak shapes. Anal. Chem. 1991, 63, 833–839. [Google Scholar] [CrossRef]








| Hex-DCM | Hex-MtBE | Hex-EtOAc | |
|---|---|---|---|
| Column and Dimensions | CHIRALPAK® IC-3, IG-3, and IK-3 (150 mm L × 4.6 mm i.d., 3 µm) | CHIRALPAK® IB N-3, IG-3, and IK-3 (150 mm L × 4.6 mm i.d., 3 µm) | CHIRALPAK® IB N-3, and IK-3 (150 mm L × 4.6 mm i.d., 3 µm) |
| Mobile Phase Ratio | 80–20 (v/v) | 80–20 (v/v) | 90–10 (v/v) |
| Flow Rate | 1.0 mL/min | ||
| Temperature | 25 °C (controlled) | ||
| Detection | 230 nm UV (for DCM and MtBE) 280 nm UV (for EtOAc) | ||
| Sample | 1.0 mg/mL in EtOH | ||
| Injection Volume | 5 µl | ||
| RT1 | RT2 | k1a | k2a | α | Rs | |
|---|---|---|---|---|---|---|
| CBC | 7.35 min | 11.08 min | 3.26 | 5.42 | 1.66 | 4.24 b |
| CBCA | 3.05 min | 3.68 min | 0.77 | 1.13 | 1.47 | 4.19 c |
| CBL | 4.34 min | 6.65 min | 1.51 | 2.85 | 1.88 | 3.40 d |
| CBLA | 2.41 min | 2.77 min | 0.40 | 0.60 | 1.53 | 2.64 e |
| Hex-DCM-TFA | Hex-MtBE-TFA | Hex-EtOAc-TFA | |
|---|---|---|---|
| Column and Dimensions | CHIRALPAK® IC-3 and IK-3 (150 mm L × 4.6 mm i.d., 3 µm) | CHIRALPAK® IK-3 (150 mm L × 4.6 mm i.d., 3 µm) | CHIRALPAK® IK-3 (150 mm L × 4.6 mm i.d., 3 µm) |
| Mobile Phase Ratio | 80–20-0.1 (v/v/v) | 80–20-0.1 (v/v/v) | 90–10-0.1 (v/v/v) |
| Flow Rate | 1.0 mL/min | ||
| Temperature | 25 °C (controlled) | ||
| Detection | 230 nm UV (for DCM and MtBE) 280 nm UV (for EtOAc) | ||
| Sample | 1.0 mg/mL in EtOH | ||
| Injection Volume | 5 µl | ||
| RT1 | RT2 | k1a | k2a | α | Rs | |
|---|---|---|---|---|---|---|
| CBC | 7.35 min | 11.08 min | 3.26 | 5.42 | 1.66 | 4.24 b |
| CBCA | 3.05 min | 3.68 min | 0.77 | 1.13 | 1.47 | 4.19 c |
| CBL | 4.34 min | 6.65 min | 1.51 | 2.85 | 1.88 | 3.40 d |
| CBLA | 2.41 min | 2.77 min | 0.40 | 0.60 | 1.53 | 2.64 e |
| RT1 | RT2 | k1a | k2a | α | Rs | |
|---|---|---|---|---|---|---|
| CBC | 5.45 min | 7.01 min | 1.00 | 1.58 | 1.58 | 5.42 b |
| CBCA | 5.31 min | 6.50 min | 0.95 | 1.39 | 1.46 | 5.05 c |
| CBL | 4.13 min | 5.24 min | 0.51 | 0.93 | 1.82 | 5.92 d |
| CBLA | 4.14 min | 4.88 min | 0.52 | 0.79 | 1.52 | 6.06 e |
| Hex−EtOH = 95−5 (v/v) | Hex−DCM = 80−20 (v/v) | Hex−EtOAc = 90−10 (v/v) | Hex−MtBE = 80−20 (v/v) | |
|---|---|---|---|---|
| CBC | n.d. | n.d. | n.d. | n.d. |
| CBCA | 28.2 mg/mL | 140.6 mg/mL | 29.1 mg/mL | 87.3 mg/mL |
| CBL | <5 mg/mL | 24.8 mg/mL | 18.7 mg/mL | 19.3 mg/mL |
| CBLA | <2 mg/mL | 4.3 mg/mL | 7.2 mg/mL | 11.4 mg/mL |
| 4.6 × 250 mm | 21 × 250 mm | 30 × 250 mm | 50 × 250 mm | kg Racemate/kg of CSP/Day | |
|---|---|---|---|---|---|
| CBC Productivity | 43.69 mg/hr | 873.8 mg/hr | 1.75 g/hr | 5.24 g/hr | 0.279 kg/kg CSP/day |
| 4.6 × 250 mm | 21 × 250 mm | 30 × 250 mm | 50 × 250 mm | kg Racemate/kg of CSP/Day | |
|---|---|---|---|---|---|
| CBCA Productivity | 15.52 mg/hr | 310.4 mg/hr | 620.8 mg/hr | 1.86 g/hr | 0.099 kg/kg CSP/day |
| 4.6 × 250 mm | 21 × 250 mm | 30 × 250 mm | 50 × 250 mm | kg Racemate/kg of CSP/Day | |
|---|---|---|---|---|---|
| CBL Productivity | 28.5 mg/hr | 570 mg/hr | 1.14 g/hr | 3.42 g/hr | 0.182 kg/kg CSP/day |
| 4.6 × 250 mm | 21 × 250 mm | 30 × 250 mm | 50 × 250 mm | kg Racemate/kg of CSP/Day | |
|---|---|---|---|---|---|
| CBLA Productivity | 9.95 mg/hr | 199 mg/hr | 398 mg/hr | 1.19 g/hr | 0.064 kg/kg CSP/day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, J.M.; Umstead, W.J. Chiral Separation of Cannabichromene, Cannabicyclol, and Their Acidic Analogs on Polysaccharide Chiral Stationary Phases. Molecules 2023, 28, 1164. https://doi.org/10.3390/molecules28031164
Ferraro JM, Umstead WJ. Chiral Separation of Cannabichromene, Cannabicyclol, and Their Acidic Analogs on Polysaccharide Chiral Stationary Phases. Molecules. 2023; 28(3):1164. https://doi.org/10.3390/molecules28031164
Chicago/Turabian StyleFerraro, John M., and Weston J. Umstead. 2023. "Chiral Separation of Cannabichromene, Cannabicyclol, and Their Acidic Analogs on Polysaccharide Chiral Stationary Phases" Molecules 28, no. 3: 1164. https://doi.org/10.3390/molecules28031164
APA StyleFerraro, J. M., & Umstead, W. J. (2023). Chiral Separation of Cannabichromene, Cannabicyclol, and Their Acidic Analogs on Polysaccharide Chiral Stationary Phases. Molecules, 28(3), 1164. https://doi.org/10.3390/molecules28031164