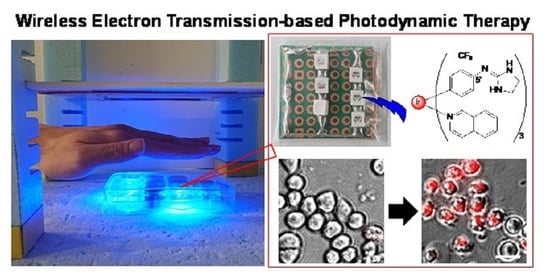
Development of Wireless Power-Transmission-Based Photodynamic Therapy for the Induction of Cell Death in Cancer Cells by Cyclometalated Iridium(III) Complexes
Abstract
:1. Introduction
2. Results and Discussions
2.1. Design and Preparation of Devices for Wireless Power-Transmission (WPT) System
2.2. Evaluation of a Power Transmitting Device and a Power Receiving Device in the WPT System
2.3. Generation of Singlet Oxygen by the Wireless Power Transmission System
2.4. Induction of Cell Death in HeLa S3 Cells upon the WPT-Based Photoirradiation of Ir Complex 7
3. Materials and Methods
3.1. General Information
3.2. Design and Evaluation of the Function of the Power Transmitting Device and Power Receiving Device for Wireless Power Transmission (WPT)
3.3. Cell Culture
3.4. Measurement of Singlet Oxygen (1O2) by Using DPBF
3.5. Photoinduced Cell Death in HeLa S3 Cells by the WPT System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References and Note
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic therapy: Past, present and future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic therapy: A compendium of latest reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: A mechanism of cancer cell cytotoxicity after near-Infrared photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef]
- Osaki, T.; Hibino, S.; Yokoe, I.; Yamaguchi, H.; Nomoto, A.; Yano, S.; Mikata, Y.; Tanaka, M.; Kataoka, H.; Okamoto, Y. A basic study of photodynamic therapy with glucose-conjugated chlorin e6 using mammary carcinoma xenografts. Cancer 2019, 11, 636. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.C.-C.; Lo, K.K.-W.J. Luminescent and photofunctional transition metal complexes: From molecular design to diagnostic and therapeutic applications. J. Am. Chem. Soc. 2022, 144, 14420–14440. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Nie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.I. Near-infrared photoimmunotherapy of cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W. (Ed.) Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef]
- Zamora, A.; Vigueras, G.; Rodríguez, V.; Santana, M.D.; Ruiz, J. Cyclometalated iridium(III) luminescent complexes in therapy and phototherapy. Coord. Chem. Rev. 2018, 360, 34–76. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, K.; Xu, G.; Liu, X.; Zhao, Q.; Xu, C.; Gou, S. An iridium (III) complex bearing a donor–acceptor–donor type ligand for NIR-triggered dual phototherapy. Adv. Funct. Mater. 2021, 31, 2008325. [Google Scholar] [CrossRef]
- Li, J.; Chen, T. Transition metal complexes as photosensitizers for integrated cancer theranostic applications. Coord. Chem. Rev. 2020, 418, 213355. [Google Scholar] [CrossRef]
- Ho, P.-Y.; Ho, C.-L.; Wong, W.-Y. Recent advances of iridium(III) metallophosphors for health-related applications. Coord. Chem. Rev. 2020, 413, 213267. [Google Scholar] [CrossRef]
- Huang, T.; Yu, Q.; Liu, S.; Huang, W.; Zhao, Q. Phosphorescent iridium(III) complexes: A versatile tool for biosensing and photodynamic therapy. Dalton Trans. 2018, 47, 7628–7633. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tan, C.; Cao, Q.; Mao, Z. Application of phosphorescent cyclometalated iridium(III) complexes in cancer treatment. Prog. Chem. 2018, 30, 1548–1556. [Google Scholar]
- Huang, H.; Banerjee, S.; Sadler, P.J. Recent advances in the design of targeted iridium(III) photosensitizers for photodynamic therapy. ChemBioChem 2018, 19, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.-C.; Huang, L.; Leung, P.K.-K.; Lo, K.K.-W. Recent development of photofunctional transition metal-peptide conjugates for bioimaging and therapeutic applications. Eur. J. Inorg. Chem. 2022, 2022, e202200455. [Google Scholar] [CrossRef]
- Moromizato, S.; Hisamatsu, Y.; Suzuki, T.; Matsuo, Y.; Abe, R.; Aoki, S. Design and synthesis of luminescent cyclometalated iridium(III) complex having N,N-diethylamino group that stains acidic intracellular organelles and induces cell death by photoirradiation. Inorg. Chem. 2012, 51, 12697–12706. [Google Scholar] [CrossRef]
- Nakagawa, A.; Hisamatsu, Y.; Moromizato, S.; Kohno, M.; Aoki, S. Synthesis of tris-cyclometalated iridium(III) complexes having pyridyl moiety by regioselective substitution reactions and their photochemical properties. Inorg. Chem. 2014, 53, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Kando, A.; Hisamatsu, Y.; Ohwada, H.; Itoh, T.; Moromizato, S.; Kohno, M.; Aoki, S. Photochemical properties of red-emitting tris(cyclometalated) iridium(III) complexes having basic and nitro groups and application to pH sensing and photoinduced cell death. Inorg. Chem. 2015, 54, 5342–5357. [Google Scholar] [CrossRef]
- Aoki, S.; Yokoi, K.; Hisamatsu, Y.; Balachandran, C.; Tamura, Y.; Tanaka, T. Post-complexation functionalization of cyclometalated iridium(III) complexes and applications to biomedical and material sciences. Top. Curr. Chem. 2022, 380, 36. [Google Scholar] [CrossRef]
- Ruggiero, E.; Castro, S.A.; Habtemariam, A.; Salassa, L. Upconverting nanoparticles for the near infrared photoactivation of transition metal complexes: New opportunities and challenges in medicinal inorganic photochemistry. Dalton Trans. 2016, 45, 13012–13020. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Swamy, P.C.A.; Sivaraman, G.; Priyamka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Khan, S.R.; Pavuluri, S.K.; Cummins, G.; Desmulliez, M.P.Y. Wireless power transfer techniques for implantable medical devices: A review. Sensor 2020, 20, 3487. [Google Scholar] [CrossRef]
- Rong, C.; Lu, C.; Zeng, Y.; Tao, X.; Liu, X.; Liu, R.; He, X.; Liu, M. A critical review of metamaterial in wireless power transfer system. IET Power Electron. 2021, 14, 1541–1559. [Google Scholar] [CrossRef]
- Okoyeigbo, O.; Olajube, A.A.; Shobayo, O.; Aligbe, A.; Ibhaze, A.E. Wireless power transfer: A review. IOP Conf. Ser. Earth Environ. 2021, 655, 012032. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, Z.; Steiger, C.; Traverso, G.; Adib, F. Enabling deep-tissue networking for miniature medical devices. In Proceedings of the 2018 Conference of the ACM Special Interest Group on Data Communication, New York, NY, USA, 20–25 August 2018; pp. 417–431. [Google Scholar]
- Meng, M.; Kiani, M. Design and Optimization of Ultrasonic Wireless Power Transmission Links for Millimeter-Sized Biomedical Implants. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Zhou, J.; Samaddar, S.; Song, S.H.; Elzey, B.D.; Thompson, D.H.; Ziaie, B. An implantable ultrasonically-powered micro-light-source (µlight) for photodynamic therapy. Sci. Rep. 2019, 9, 1395. [Google Scholar] [CrossRef]
- Kurs, A.; Karalis, A.; Moffatt, R.; Joannopoulos, J.D.; Fisher, P.; Soljačić, M. Wireless power transfer via strongly coupled magnetic resonances. Science 2007, 317, 83–86. [Google Scholar] [CrossRef]
- Imura, T.; Hori, Y. Maximizing air gap and efficiency of magnetic resonant coupling for wireless power transfer using equivalent circuit and Neumann formula. IEEE Trans. Ind. Electron. 2011, 58, 4746–4752. [Google Scholar] [CrossRef]
- Theodoridis, M.P. Effective capacitive power transfer. IEEE Trans. Power Electron. 2012, 27, 4906–4913. [Google Scholar] [CrossRef]
- Li, S.; Mi, C.C. Wireless power transfer for electric vehicle applications. IEEE J. Emerg. Sel. Topics Power Electron. 2014, 3, 4–17. [Google Scholar]
- Okasili, I.; Elkhateb, A.; Littler, T. A review of wireless power transfer systems for electric vehicle battery charging with a focus on inductive coupling. Electronics 2022, 11, 1355. [Google Scholar] [CrossRef]
- Sasaki, K.; Imura, T. Combination of Sensorless Energized Section Switching System and Double-LCC for DWPT. In Proceedings of the 2020 IEEE PELS, Workshop on Emerging Technologies: Wireless Power Transfer (WoW), Seoul, Republic of Korea, 15–19 November 2020; pp. 62–67. [Google Scholar] [CrossRef]
- Hanawa, K.; Imura, T.; Abe, N. Basic Evaluation of Electrical Characteristics of Ferrite-less and Capacitor-less Coils by Road Embedment Experiment for Dynamic Wireless Power Transfer. In Proceedings of the 2021 IEEE PELS Workshop on Emerging Technologies: Wireless Power Transfer (WoW), San Diego, CA, USA, 1–4 June 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Choi, K.W.; Aziz, A.A.; Setiawan, D.; Tran, N.M.; Ginting, L.; Kim, D.I. Distributed wireless power transfer system for internet of thins devices. IEEE Internet Things J. 2018, 5, 2657–2671. [Google Scholar] [CrossRef]
- Rana, M.M.; Xiang, W.; Wang, E.; Li, X.; Choi, B.J. Internet of things infrastructure for wireless power transfer systems. IEEE Access 2018, 6, 19295–19303. [Google Scholar] [CrossRef]
- Kim, H.J.; Hirayama, H.; Kim, S.; Han, K.J.; Zhang, R.; Choi, J.W. Review of near-field wireless power and communication for biomedical applications. IEEE Access 2017, 5, 21264–21285. [Google Scholar] [CrossRef]
- Moore, J.; Castellanos, S.; Xu, S.; Wood, B.; Ren, H.; Tse, Z.T.H. Applications of wireless power transfer in medicine: State-of-the-art reviews. Ann. Biomed. Eng. 2019, 47, 22–38. [Google Scholar] [CrossRef]
- Bansal, A.; Yang, F.; Xi, T.; Zhang, Y.Y.; Ho, J.S. In vivo wireless photonic photodynamic therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 1469–1474. [Google Scholar] [CrossRef]
- Yamagishi, K.; Kirino, I.; Takahashi, I.; Amano, H.; Takeoka, S.; Morimoto, Y.; Fujie, T. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 2019, 3, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.M.; Tian, X.; Ho, J.S. Wireless power transfer for glioblastoma photodynamic therapy. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar]
- Kim, W.S.; Khot, M.I.; Woo, H.-M.; Hong, S.; Baek, D.-H.; Maisey, T.; Daniels, B.; Coletta, P.L.; Yoon, B.-J.; Jayne, D.G.; et al. AI-enabled implantable, multichannel wireless telemetry for photodynamic therapy. Nat. Commun. 2022, 13, 2178. [Google Scholar] [CrossRef]
- In the initial PRs, 2 LEDs were connected. However, the efficiency of the cell death induction was low. Therefore, we decided to use 6 LEDs in one PR device for more efficient photo-induced cell death in cancer cells
- Howard, J.A.; Mendenhall, G.D. Autoxidation and photooxidation of 1,3-diphenylisobenzofuran: A kinetic and product study. Can. J. Chem. 1975, 53, 2199–2201. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoi, K.; Yasuda, Y.; Kanbe, A.; Imura, T.; Aoki, S. Development of Wireless Power-Transmission-Based Photodynamic Therapy for the Induction of Cell Death in Cancer Cells by Cyclometalated Iridium(III) Complexes. Molecules 2023, 28, 1433. https://doi.org/10.3390/molecules28031433
Yokoi K, Yasuda Y, Kanbe A, Imura T, Aoki S. Development of Wireless Power-Transmission-Based Photodynamic Therapy for the Induction of Cell Death in Cancer Cells by Cyclometalated Iridium(III) Complexes. Molecules. 2023; 28(3):1433. https://doi.org/10.3390/molecules28031433
Chicago/Turabian StyleYokoi, Kenta, Yoshitaka Yasuda, Azusa Kanbe, Takehiro Imura, and Shin Aoki. 2023. "Development of Wireless Power-Transmission-Based Photodynamic Therapy for the Induction of Cell Death in Cancer Cells by Cyclometalated Iridium(III) Complexes" Molecules 28, no. 3: 1433. https://doi.org/10.3390/molecules28031433
APA StyleYokoi, K., Yasuda, Y., Kanbe, A., Imura, T., & Aoki, S. (2023). Development of Wireless Power-Transmission-Based Photodynamic Therapy for the Induction of Cell Death in Cancer Cells by Cyclometalated Iridium(III) Complexes. Molecules, 28(3), 1433. https://doi.org/10.3390/molecules28031433