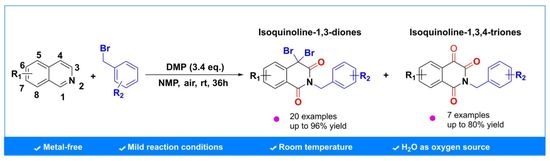
Dess–Martin Periodinane-Mediated Oxidative Coupling Reaction of Isoquinoline with Benzyl Bromide
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Procedure for the Oxidative Coupling Reactions
- 2-Benzyl-4,4-dibromoisoquinoline-1,3(2H,4H)-dione (3aa). White solid, 193 mg; mp: 116 °C; IR (KBr) cm−1 2965, 1679, 1597, 1429, 1375, 1336, 1271, 1238, 1150, 960,731, 699; 1H NMR (400 MHz, CDCl3) δ 8.14 (t, J = 7.6 Hz, 2H), 7.75 (t, J = 7.8 Hz, 1H), 7.54 (t, J = 7.7 Hz, 1H), 7.48 (d, J = 7.4 Hz, 2H), 7.30 (dt, J = 19.2, 7.2 Hz, 3H), 5.26 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 165.0, 161.5, 140.3, 135.4, 134.6, 130.4, 130.2, 128.7, 128.3, 127.6, 120.1, 44.9; ESI-HRMS: m/z calcd for C16H11Br2NO2Na [M + Na]+: 429.90542, found 429.90537.
- 4,4-Dibromo-2-(3-methylbenzyl)isoquinoline-1,3(2H,4H)-dione (3ab). White solid, 190 mg; mp: 95 °C; IR (KBr) cm−1 2960, 1724, 1681, 1598, 1428, 1371, 1347, 1235, 1150, 960, 748, 696; 1H NMR (400 MHz, CDCl3) δ 8.14 (ddd, J = 8.1, 7.1, 1.3 Hz, 2H), 7.74 (td, J = 7.7, 1.4 Hz, 1H), 7.53 (td, J = 7.6, 1.2 Hz, 1H), 7.28 (dd, J = 8.9, 1.9 Hz, 2H), 7.21 (t, J = 7.5 Hz, 1H), 7.09 (d, J = 7.5 Hz, 1H), 5.23 (s, 2H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.3, 161.9, 140.6, 138.3, 135.7, 134.9, 130.7, 130.5, 129.6, 128.7, 128.6, 128.5, 125.9, 120.5, 51.0, 45.2, 21.5. ESI-HRMS: m/z calcd for C17H13Br2NO2Na [M + Na]+: 443.92107, found 443.92127.
- 4,4-Dibromo-2-(4-methylbenzyl)isoquinoline-1,3(2H,4H)-dione (3ac). White solid, 201 mg; mp: 92 °C; IR (KBr) cm−1 2960, 1726, 1690, 1598, 1438, 1372, 1337, 1307, 1237, 1154, 964, 746, 692; 1H NMR (400 MHz, CDCl3) δ 8.18–8.09 (m, 2H), 7.74 (td, J = 7.7, 1.5 Hz, 1H), 7.53 (td, J = 7.6, 1.3 Hz, 1H), 7.39 (d, J = 7.8 Hz, 2H), 7.13 (d, J = 7.8 Hz, 2H), 5.22 (s, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.0, 161.5, 140.3, 137.3, 134.5, 132.5, 130.4, 130.1, 128.9, 128.7, 128.3, 120.2, 44.6, 20.9; ESI-HRMS: m/z calcd for C17H13Br2NO2Na [M + Na]+: 443.92107, found 443.92129.
- 4,4-Dibromo-2-(3-methoxybenzyl)isoquinoline-1,3(2H,4H)-dione (3ad). White solid, 197 mg; mp: 75 °C; IR (KBr) cm−1 2962, 1724, 1683, 1609, 1508, 1457, 1376, 1333, 1246, 1183, 1026, 951, 748, 689; 1H NMR (400 MHz, CDCl3) δ 8.14 (td, J = 8.4, 1.3 Hz, 2H), 7.75 (td, J = 7.7, 1.5 Hz, 1H), 7.54 (td, J = 7.7, 1.2 Hz, 1H), 7.28–7.19 (m, 1H), 7.05 (d, J = 7.6 Hz, 1H), 7.01 (t, J = 2.1 Hz, 1H), 6.82 (dd, J = 8.1, 2.6 Hz, 1H), 5.23 (s, 2H), 3.78 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.0, 161.5, 159.4, 140.3, 136.9, 134.6, 130.5, 130.2, 129.3, 128.3, 120.8, 120.0, 113.8, 113.4, 54.9, 44.7; ESI-HRMS: m/z calcd for C17H13Br2NO2Na [M + Na]+: 459.91599, found 459.91580.
- 4,4-Dibromo-2-(4-methoxybenzyl)isoquinoline-1,3(2H,4H)-dione (3ae). White solid, 211 mg; mp: 63 °C; IR (KBr) cm−1 2971, 1725, 1686, 1599, 1465, 1375, 1330, 1265, 1235, 1167, 1036, 955, 742, 712; 1H NMR (400 MHz, CDCl3) δ 8.13 (ddd, J = 7.9, 4.6, 1.2 Hz, 2H), 7.74 (td, J = 7.7, 1.4 Hz, 1H), 7.53 (td, J = 7.7, 1.2 Hz, 1H), 7.49–7.41 (m, 2H), 6.93–6.80 (m, 2H), 5.19 (s, 2H), 3.77 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.0, 161.5, 159.0, 140.2, 134.5, 130.4, 130.2, 130.1, 129.1, 128.2, 127.6, 120.2, 113.6, 113.5, 54.9, 44.4; ESI-HRMS: m/z calcd for C17H13Br2NO2Na [M + Na]+: 459.91599, found 459.91613.
- 4,4-Dibromo-2-(3-chlorobenzyl)isoquinoline-1,3(2H,4H)-dione (3af). Yellow oil, 190 mg; mp: 85 °C; IR (KBr) cm−1 2920, 1720, 1670, 1595, 1419, 1369, 1338, 1266, 1236, 1158, 966, 748, 708; 1H NMR (400 MHz, CDCl3) δ 8.15 (td, J = 8.4, 1.3 Hz, 2H), 7.76 (td, J = 7.7, 1.4 Hz, 1H), 7.55 (td, J = 7.6, 1.2 Hz, 1H), 7.47 (q, J = 1.4 Hz, 1H), 7.37 (td, J = 4.7, 1.7 Hz, 1H), 7.32–7.16 (m, 2H), 5.22 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 165.0, 161.5, 140.3, 137.9, 135.3, 134.6, 130.4, 130.1, 129.3, 128.4, 128.3, 128.2, 125.6, 120.2, 44.9; ESI-HRMS: m/z calcd for C16H10Br2ClNO2Na [M + Na]+: 463.86645, found 463.86648.
- 4,4-Dibromo-2-(3-bromobenzyl)isoquinoline-1,3(2H,4H)-dione (3ag). Yellow oil, 187 mg; mp: 75 °C; IR (KBr) cm−1 3074, 1727, 1671, 1595, 1436, 1376, 1336, 1272, 1234, 1153, 960, 884, 745, 708; 1H NMR (400 MHz, CDCl3) δ 8.13 (ddd, J = 10.3, 8.0, 1.3 Hz, 2H), 7.75 (td, J = 7.7, 1.4 Hz, 1H), 7.62 (t, J = 1.9 Hz, 1H), 7.54 (td, J = 7.6, 1.1 Hz, 1H), 7.44–7.36 (m, 2H), 7.18 (t, J = 7.9 Hz, 1H), 5.20 (s, 2H).13C NMR (100 MHz, CDCl3) δ 164.9, 161.4, 140.1, 137.5, 134.7, 131.5, 130.8, 130.5, 130.1, 129.8, 128.3, 127.2, 122.2, 119.9, 44.1. ESI-HRMS: m/z calcd for C16H10Br3NO2Na [M + Na]+: 507.81694, found 507.81615.
- 4,4-Dibromo-2-(4-nitrobenzyl)isoquinoline-1,3(2H,4H)-dione (3ah). White solid, 211 mg; mp: 133 °C; IR (KBr) cm−1 3061, 1725, 1686, 1600, 1517, 1430, 1380, 1345, 1235, 954, 705; 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 8.21–8.11 (m, 3H), 7.79 (td, J = 7.8, 1.4 Hz, 1H), 7.68–7.61 (m, 2H), 7.57 (td, J = 7.6, 1.1 Hz, 1H), 5.33 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 165.1, 161.6, 147.4, 142.4, 140.2, 135.0, 130.69, 130.3, 129.4, 128.4, 123.6, 119.7, 44.1; ESI-HRMS: m/z calcd for C16H11Br2N2O4 [M + H]+: 452.90856, found 452.90488.
- 2-Allyl-4,4-dibromoisoquinoline-1,3(2H,4H)-dione (3ai). Colorless oil, 110 mg; mp: 63 °C; IR (KBr) cm−1 2938, 1717, 1683, 1597, 1459, 1420, 1373, 1345, 1239, 1163, 965, 753, 699; 1H NMR (400 MHz, CDCl3) δ 8.14 (ddd, J = 10.8, 8.0, 1.3 Hz, 2H), 7.75 (td, J = 7.7, 1.4 Hz, 1H), 7.54 (td, J = 7.6, 1.2 Hz, 1H), 5.91 (ddt, J = 17.3, 10.2, 5.8 Hz, 1H), 5.34 (dq, J = 17.1, 1.5 Hz, 1H), 5.24 (dq, J = 10.3, 1.3 Hz, 1H), 4.66 (dt, J = 5.9, 1.4 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 164.6, 161.2, 140.3, 134.5, 130.4, 130.3, 130.2, 128.2, 120.0, 118.5, 43.6; ESI-HRMS: m/z calcd for C12H10Br2NO2 [M + H]+: 357.90783, found 357.90736.
- 4,4-Dibromo-2-(naphthalen-2-ylmethyl)isoquinoline-1,3(2H,4H)-dione (3aj). White solid, 164 mg; mp: 112 °C; IR (KBr) cm−1 3043, 1718, 1677, 1599, 1431, 1336, 1235, 1151, 975, 748, 597; 1H NMR (400 MHz, CDCl3) δ 8.15 (dt, J = 7.8, 1.7 Hz, 2H), 7.99–7.94 (m, 1H), 7.87–7.77 (m, 3H), 7.74 (td, J = 7.7, 1.4 Hz, 1H), 7.61 (dd, J = 8.5, 1.8 Hz, 1H), 7.53 (td, J = 7.7, 1.1 Hz, 1H), 7.50–7.40 (m, 2H), 5.43 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 165.1, 161.6, 140.2, 134.6, 132.9, 132.8, 132.6, 130.4, 130.1, 128.3, 128.1, 127.9, 127.7, 127.3, 126.4, 125.9, 125.8, 120.1, 45.0; ESI-HRMS: m/z calcd for C20H13Br2NO2Na [M + Na]+: 479.92107, found 479.92071.
- 2-Benzyl-4,4-dibromo-6-methylisoquinoline-1,3(2H,4H)-dione (3ba). White solid, 193 mg; mp: 105 °C; IR (KBr) cm−1 2923, 1717, 1676, 1608, 1419, 1374, 1348, 1246, 971, 716, 699; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.1 Hz, 1H), 7.95–7.90 (m, 1H), 7.51–7.44 (m, 2H), 7.37–7.22 (m, 4H), 5.24 (s, 2H), 2.51 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.1, 161.5, 145.9, 140.1, 135.5, 131.5, 130.4, 128.6, 128.3, 128.3, 127.6, 117.6, 44.7, 21.6; ESI-HRMS: m/z calcd for C17H14Br2NO2 [M + H]+: 421.93913, found 421.93872.
- 4,4-Dibromo-2-(3-bromobenzyl)-6-methylisoquinoline-1,3(2H,4H)-dione (3bb). White solid, 198 mg; mp: 115 °C; IR (KBr) cm−1 2917, 1724, 1675, 1608, 1433, 1331, 1225, 1152, 956, 685; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.1 Hz, 1H), 7.94 (s, 1H), 7.61 (d, J = 1.9 Hz, 1H), 7.40 (dd, J = 7.9, 1.8 Hz, 2H), 7.34 (dd, J = 8.1, 1.6 Hz, 1H), 7.19 (t, J = 7.9 Hz, 1H), 5.19 (s, 2H), 2.52 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.1, 161.5, 146.1, 140.1, 137.6, 131.6, 130.8, 130.4, 129.8, 128.4, 127.2, 122.3, 117.4, 44.1, 21.6; ESI-HRMS: m/z calcd for C17H12Br3NO2 Na [M + Na]+: 521.83159, found 521.83128.
- 4,4-Dibromo-6-methyl-2-(3-methylbenzyl)isoquinoline-1,3(2H,4H)-dione (3bc). White solid, 178 mg; mp: 92 °C; IR (KBr) cm−1 2922, 1721, 1676, 1609, 1422, 1369, 1341, 1248, 1158, 971, 730, 696; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.1 Hz, 1H), 7.93 (s, 1H), 7.38–7.16 (m, 4H), 7.08 (d, J = 7.4 Hz, 1H), 5.21 (s, 2H), 2.51 (s, 3H), 2.32 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.1, 161.5, 145.9, 140.2, 137.9, 135.4, 131.5, 130.4, 129.2, 128.4, 128.3, 128.2, 125.6, 117.7, 44.7, 21.6, 21.1; ESI-HRMS: m/z calcd for C18H15Br2NO2Na [M + Na]+:2 457.93672, found 457.93633.
- 2-Benzyl-4,4,6-tribromoisoquinoline-1,3(2H,4H)-dione (3bd). White solid, 218 mg; mp: 112 °C; IR (KBr) cm−1 3063, 1721, 1679, 1585, 1406, 1344, 1239, 1159, 971, 727, 693; 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 1.8 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.66 (dd, J = 8.4, 1.8 Hz, 1H), 7.50–7.42 (m, 2H), 7.39–7.23 (m, 3H), 5.24 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 164.4, 160.9, 141.6, 135.2, 133.9, 133.0, 129.8, 129.7, 128.7, 128.3, 127.7, 118.9, 45.0; ESI-HRMS: m/z calcd for C16H11Br3NO2 [M + H]+: 485.83399, found 485.83301.
- 4,4,6-Tribromo-2-(3-bromobenzyl)isoquinoline-1,3(2H,4H)-dione (3be). Colorless oil, 219 mg; mp: 81 °C; IR (KBr) cm−1 2921, 1723, 1683, 1586, 1472, 1403, 1336, 1236, 1158, 969, 756, 693; 1H NMR (400 MHz, CDCl3) δ 8.29 (d, J = 1.8 Hz, 1H), 7.99 (s, 1H), 7.68 (dd, J = 8.4, 1.8 Hz, 1H), 7.61 (q, J = 3.1, 2.5 Hz, 1H), 7.41 (td, J = 7.8, 1.7 Hz, 2H), 7.20 (t, J = 7.9 Hz, 1H), 5.19 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 164.5, 160.9, 141.6, 137.3, 134.0, 133.1, 131.7, 131.0, 129.9, 129.9, 127.4, 122.4, 118.8, 44.3; ESI-HRMS: m/z calcd for C16H9Br4O2Na [M + Na]+: 585.72654, found 585.76965.
- 4,4,6-Tribromo-2-(3-methylbenzyl)isoquinoline-1,3(2H,4H)-dione (3bf). Colorless oil, 200 mg; mp: 108 °C; IR (KBr) cm−1 2922, 1720, 1682, 1586, 1405, 1339, 1234, 1161, 970, 759, 689; 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 1.9 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.66 (dd, J = 8.4, 1.8 Hz, 1H), 7.32–7.11 (m, 4H), 7.09 (d, J = 7.6 Hz, 1H), 5.20 (s, 2H), 2.32 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.5, 160.9, 141.6, 138.0, 135.1, 133.9, 133.0, 129.8, 129.7, 129.3, 128.7, 128.5, 128.2, 125.6, 119.0, 45.0, 21.1; ESI-HRMS: m/z calcd for C17H12Br3O2Na [M + Na]+: 521.83159, found 521.83128.
- 2-Benzyl-4,4,7-tribromoisoquinoline-1,3(2H,4H)-dione (3bg). White solid, 223 mg; mp: 163 °C; IR (KBr) cm−1 2922, 1702, 1670, 1588, 1432, 1348, 1259, 1148, 961, 696; 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 2.1 Hz, 1H), 8.01 (d, J = 8.5 Hz, 1H), 7.85 (dd, J = 8.5, 2.1 Hz, 1H), 7.50–7.42 (m, 2H), 7.39–7.23 (m, 3H), 5.24 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 164.6, 160.5, 139.0, 137.6, 135.1, 131.8, 131.0, 128.7, 128.4, 127.8, 124.9, 121.5, 45.1; ESI-HRMS: m/z calcd for C16H10Br3NO2Na [M + Na] +: 507.81594, found 507.27163.
- 4,4,7-Tribromo-2-(3-methylbenzyl)isoquinoline-1,3(2H,4H)-dione (3bh). Colorless oil, 199 mg; mp: 127 °C; IR (KBr) cm−1 2925, 1683, 1599, 1430, 1320, 1297, 1245, 1020, 687; 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 2.1 Hz, 1H), 8.01 (d, J = 8.5 Hz, 1H), 7.84 (dd, J = 8.5, 2.1 Hz, 1H), 7.26 (d, J = 9.1 Hz, 2H), 7.21 (t, J = 7.4 Hz, 1H), 7.09 (d, J = 7.4 Hz, 1H), 5.21 (s, 2H), 2.33 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.5, 160.4, 139.0, 138.0, 137.6, 135.0, 131.8, 131.0, 129.3, 128.7, 128.5, 128.2, 125.6, 124.9, 121.5, 49.5, 45.0, 21.1; ESI-HRMS: m/z calcd for C17H12Br3O2Na [M + Na]+: 521.83159, found 521.83128.
- 2-Benzyl-4,4-dibromo-6-chloroisoquinoline-1,3(2H,4H)-dione (3bi). Yellow solid, 88 mg; mp: 88 °C; IR (KBr) cm−1 3087, 1730, 1688, 1587, 1426, 1374, 1349, 1264, 1230, 906, 733, 706; 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 7.6, 1.6 Hz, 1H), 7.85–7.72 (m, 2H), 7.52–7.46 (m, 2H), 7.35–7.23 (m, 4H), 5.21 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 172.4, 161.0, 156.3, 137.2, 136.1, 135.3, 135.1, 132.0, 129.2, 128.9, 128.4, 127.9, 127.22, 44.3; ESI-HRMS: m/z calcd for C16H10ClNO3Na [M + Na]+: 322.02469, found 322.02361.
- 4,4-Dibromo-6-chloro-2-(3-methylbenzyl)isoquinoline-1,3(2H,4H)-dione (3bj). White solid, 72 mg; mp: 108 °C; IR (KBr) cm−1 2921, 1729, 1682, 1589, 1416, 1326, 1266, 1225, 1156, 1077, 976, 906, 764, 707; 1H NMR (400 MHz, CDCl3) δ 7.95 (dd, J = 7.7, 1.0 Hz, 1H), 7.77 (dd, J = 8.5, 7.8 Hz, 1H), 7.52–7.47 (m, 2H), 7.34–7.26 (m, 4H), 5.19 (s, 2H), 4.02 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 172.2, 161.9, 160.8, 157.0, 136.7, 135.6, 131.5, 129.1, 128.3, 127.7, 121.9, 118.9, 117.3, 56.5, 44.1; ESI-HRMS: m/z calcd for C17H14NO4 [M + H]+: 296.09228, found 296.09301.
- 2-Benzyl-5-bromoisoquinoline-1,3,4(2H)-trione (3ca). Colorless oil, 139 mg; mp: 158 °C; IR (KBr) cm−1 2923, 1705, 1669, 157, 1427, 1361, 1237, 1163, 1025, 950, 733, 698; 1H NMR (400 MHz, CDCl3) δ 8.07 (dd, J = 8.9, 1.8 Hz, 1H), 7.57 (d, J = 2.4 Hz, 1H), 7.51–7.44 (m, 2H), 7.39–7.29 (m, 2H), 7.33–7.22 (m, 2H), 7.03 (dt, J = 8.8, 2.2 Hz, 1H), 5.23 (s, 2H), 3.96 (d, J = 1.8 Hz, 3H), 1.37–1.20 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 165.1, 164.3, 161.2, 142.3, 135.6, 130.6, 128.6, 128.3, 127.5, 117.2, 114.3, 112.9, 55.7, 44.7, 29.4; ESI-HRMS: m/z calcd for C17H13NO4Na [M + Na]+: 318.07423, found 318.07315.
- 2-Benzyl-5-chloroisoquinoline-1,3,4(2H)-trione (3cb). White solid, 81 mg; mp: 193 °C; IR (KBr) cm−1 2922, 1700, 1674, 1574, 1433, 1362, 1332, 1235, 1153, 102, 753, 703; 1H NMR (400 MHz, CDCl3) δ 8.15 (dt, J = 7.8, 1.7 Hz, 2H), 7.99–7.94 (m, 1H), 7.87–7.77 (m, 3H), 7.74 (td, J = 7.7, 1.4 Hz, 1H), 7.61 (dd, J = 8.5, 1.8 Hz, 1H), 7.53 (td, J = 7.7, 1.1 Hz, 1H), 7.50–7.40 (m, 2H), 5.43 (s, 2H), 0.09 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 165.1, 161.6, 140.2, 134.6, 132.9, 132.8, 132.6, 130.4, 130.1, 128.3, 128.1, 127.9, 127.7, 127.3, 126.4, 125.9, 125.8, 120.1, 45.0; ESI-HRMS: m/z calcd for C16H10ClNO3Na [M + Na]+: 322.02469, found 322.02414.
- 2-Benzyl-5-methoxyisoquinoline-1,3,4(2H)-trione (3cc). Yellow solid, 100 mg; mp: 183 °C; IR (KBr) cm−1 2924, 1723, 1680, 1587, 1453, 1379, 1358, 1283, 1225, 1070, 1014, 751, 699; 1H NMR (400 MHz, CDCl3) δ 8.39 (dd, J = 7.8, 1.1 Hz, 1H), 8.04 (dd, J = 8.1, 1.1 Hz, 1H), 7.66 (t, J = 7.9 Hz, 1H), 7.52–7.47 (m, 2H), 7.36–7.27 (m, 3H), 5.22 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 165.6, 163.2, 160.8, 141.9, 140.7, 135.3, 133.94, 132.7, 130.8, 129.3, 128.3, 128.2, 127.9, 127.5, 125.3, 120.0, 44.7; ESI-HRMS: m/z calcd for C16H10BrNO3Na [M + Na]+: 365.97418, found 365.97343.
- 2-Benzyl-6-methoxyisoquinoline-1,3,4(2H)-trione (3cd). Colorless oil, 123 mg; mp: 135 °C; IR (KBr) cm−1 2924, 1695, 1674, 1593, 1466, 1286, 1008, 872, 757, 622; 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 8.8 Hz, 1H), 7.57 (d, J = 2.5 Hz, 1H), 7.26 (d, J = 9.0 Hz, 2H), 7.20 (t, J = 7.5 Hz, 1H), 7.11–7.00 (m, 2H), 5.20 (s, 2H), 3.96 (s, 3H), 2.32 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.1, 164.3, 161.2, 143.2, 137.9, 135.5, 135.7, 130.7, 129.2, 128.3, 128.2, 125.5, 117.2, 114.3, 113.0, 55.7, 44.7, 26.7; ESI-HRMS: m/z calcd for C17H13NO4Na [M + Na]+: 332.08988, found 332.09010.
- 2-(3-Bromobenzyl)-6-methoxyisoquinoline-1,3,4(2H)-trione (3ce). White solid, 121 mg; mp: 102 °C; IR (KBr) cm−1 2967, 1723, 1677, 1604, 1499, 1432, 1336, 1372, 1288, 1245, 1152, 1026, 960, 740, 691; 1H NMR (400 MHz, CDCl3) δ 8.07 (dd, J = 8.8, 1.8 Hz, 1H), 7.57 (d, J = 2.4 Hz, 1H), 7.54–7.46 (m, 2H), 7.36–7.25 (m, 3H), 7.03 (dd, J = 8.8, 2.4 Hz, 1H), 5.23 (s, 2H), 3.96 (d, J = 1.7 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 165.1, 164.3, 161.2, 142.3, 135.6, 130.6, 128.6, 128.3, 127.5, 117.2, 114.3, 112.9, 55.7, 44.7. ESI-HRMS: m/z calcd for C17H13NO4Na [M + Na]+: 318.07423, found 318.07493.
- 6-Methoxy-2-(3-methylbenzyl)isoquinoline-1,3,4(2H)-trione (3cf). Colorless oil, 148 mg; mp: 112 °C; IR (KBr) cm−1 2923, 1653, 1600, 1457, 1403, 1254, 1206, 1130, 1093, 1024, 944, 766; 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 8.9 Hz, 1H), 7.56 (dd, J = 11.6, 2.1 Hz, 2H), 7.36 (t, J = 6.5 Hz, 2H), 7.15 (t, J = 7.8 Hz, 1H), 7.01 (dd, J = 8.9, 2.5 Hz, 1H), 5.15 (s, 2H), 3.92 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.9, 164.3, 160.9, 142.1, 137.7, 131.3, 130.6, 130.5, 129.7, 127.1, 122.1, 117.1, 114.3, 112.6, 55.6, 50.7, 43.84; ESI-HRMS: m/z calcd for C17H12BrNO4Na [M + Na]+: 395.98474, found 395.98453.
- 2-Benzyl-7-methoxyisoquinoline-1,3,4(2H)-trione (3cg). Colorless oil, 113 mg; mp: 109 °C; IR (KBr) cm−1 2925, 1708, 1675, 1603, 1497, 1429, 1376, 1341, 1280, 1244, 1115, 1026, 961, 763, 698; 1H NMR (400 MHz, CDCl3) δ 7.99 (dd, J = 8.0, 1.2 Hz, 1H), 7.83 (dd, J = 7.8, 1.7 Hz, 1H), 7.52–7.45 (m, 2H), 7.45–7.30 (m, 4H), 7.14 (td, J = 7.7, 1.8 Hz, 1H), 5.39 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 165.9, 141.0, 135.1, 134.5, 132.4, 130.7, 128.3, 128.2, 128.1, 127.6, 93.9, 67.0; ESI-HRMS: m/z calcd for C16H11Br2NONa [M + Na]+: 391.92855, found 391.92857.
4.3. X-ray Crystallographic Data of 3aa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, D.; Yu, P.; Xiao, W.; Wang, Z.Z.; Zhao, L.G. Berberine: A Promising Natural Isoquinoline Alkaloid for the Development of Hypolipidemic Drugs. Curr. Top. Med. Chem. 2020, 20, 2634–2647. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. Int. J. Mol. Sci. 2021, 22, 1653. [Google Scholar] [CrossRef] [PubMed]
- Chueh, W.H.; Lin, J.Y. Berberine, an Isoquinoline Alkaloid in Herbal Plants, Protects Pancreatic Islets and Serum Lipids in Nonobese Diabetic Mice. J. Agric. Food Chem. 2011, 59, 8021–8027. [Google Scholar] [CrossRef]
- Hua, R. Isoquinolone Syntheses by Annulation Protocols. Catalysts 2021, 11, 620. [Google Scholar] [CrossRef]
- Krane, B.D.; Shamma, M. The Isoquinolone Alkaloids. J. Nat. Prod. 1982, 45, 377–384. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Roy, P.; Sondhi, S.M.; Sharma, A. Synthesis of Acridine Cyclic Imide Hybrid Molecules and Their Evaluation for Anticancer Activity. Med. Chem. Res. 2015, 24, 3272–3282. [Google Scholar] [CrossRef]
- Braña, M.F.; Cacho, M.; García, M.A.; De Pascual Teresa, B.; Ramos, A.; Domínguez, M.T.; Pozuelo, J.M.; Abradelo, C.; Rey Stolle, M.F.; Yuste, M.; et al. New Analogues of Amonafide and Elinafide, Containing Aromatic Heterocycles: Synthesis, Antitumor Activity, Molecular Modeling, and DNA Binding Properties. J. Med. Chem. 2004, 47, 1391–1399. [Google Scholar] [CrossRef]
- Malaisse, W.J. Gliquidone Contributes to Improvement of Type 2 Diabetes Mellitus Management: A Review of Pharmacokinetic and Clinical Trial Data. Drugs R D 2006, 7, 331–337. [Google Scholar] [CrossRef]
- Jang, Y.; Han, J.; Li, X.; Shin, H.; Cho, W.J.; Kim, M. Antiviral Activity of Isoquinolone Derivatives against Influenza Viruses and Their Cytotoxicity. Pharmaceuticals 2021, 14, 650. [Google Scholar] [CrossRef]
- Mayer, S.C.; Banker, A.L.; Boschelli, F.; Di, L.; Johnson, M.; Kenny, C.H.; Krishnamurthy, G.; Kutterer, K.; Moy, F.; Petusky, S.; et al. Lead Identification to Generate Isoquinolinedione Inhibitors of Insulin-like Growth Factor Receptor (IGF-1R) for Potential Use in Cancer Treatment. Bioorg. Med. Chem. Lett. 2008, 18, 3641–3645. [Google Scholar] [CrossRef]
- Francis, S.; Croft, D.; Schüttelkopf, A.W.; Parry, C.; Pugliese, A.; Cameron, K.; Claydon, S.; Drysdale, M.; Gardner, C.; Gohlke, A.; et al. Structure-Based Design, Synthesis and Biological Evaluation of a Novel Series of Isoquinolone and Pyrazolo[4,3-c] Pyridine Inhibitors of Fascin 1 as Potential Anti-Metastatic Agents. Bioorg. Med. Chem. Lett. 2019, 29, 1023–1029. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Z.; Hua, J.; Lin, Y.; Bian, M.; Li, Y.; Liu, C.; He, W.; Fang, Z.; Guo, K. The Continuous-Flow Electrosynthesis of 4-(Sulfonylmethyl) Isoquinoline-1,3(2H, 4H)-Diones from N -Alkyl- N -Methacryloyl Benzamides under Metal-Free and Oxidant-Free Conditions. Org. Chem. Front. 2020, 7, 3223–3228. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, R.; Xue, W.; Liu, X.; Zhao, Y.; Wang, K.H.; Huang, D.; Huo, C.; Hu, Y. Visible-Light-Promoted Acyl Radical Cascade Reaction for Accessing Acylated Isoquinoline-1,3(2: H,4 H)-Dione Derivatives. Org. Biomol. Chem. 2020, 18, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Zhang, H.; Liu, D.; Gu, M.; Li, J.; Wu, F.; Zhu, X.; Li, J.; Nan, F. Design, Synthesis, and Biological Evaluation of Isoquinoline-1, 3, 4-Trione Derivatives as Potent. J. Med. Chem. 2006, 49, 1613–1623. [Google Scholar] [CrossRef]
- Tang, S.; Deng, Y.L.; Li, J.; Wang, W.X.; Ding, G.L.; Wang, M.W.; Xiao, Z.P.; Wang, Y.C.; Sheng, R.L. Synthesis of Perfluorinated Isoquinolinediones through Visible-Light-Induced Cyclization of Alkenes. J. Org. Chem. 2015, 80, 12599–12605. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, S.; Fang, X.; Liu, P.; Sun, P. Addition of Nitrogen Dioxide to Carbon-Carbon Double Bond Followed by a Cyclization to Construct Nitromethylated Isoquinolinediones. Org. Biomol. Chem. 2017, 15, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Luo, W.K.; Yang, L.; Ma, D.Y. Iodine-Catalyzed Oxidative Multiple C-H Bond Functionalization of Isoquinolines with Methylarenes: An Efficient Synthesis of Isoquinoline-1,3,4(2: H)-Triones. Org. Biomol. Chem. 2017, 15, 7112–7116. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Du, D.M. Diastereoselective Construction of CF3-Containing Bispiro[Isoquinolone-Pyrrolidine-Benzothiophenone]s through 1,3-Dipolar Cycloaddition. Eur. J. Org. Chem. 2022, 2022, e202200645. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Basak, A.K.; Venkat Narsaiah, A. Recyclable 2nd Generation Ionic Liquids as Green Solvents for the Oxidation of Alcohols with Hypervalent Iodine Reagents. Tetrahedron 2004, 60, 2131–2135. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Muniz, K. Editorial for the Special Issue on Hypervalent Iodine Reagents. J. Org. Chem. 2017, 82, 11667–11668. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Xu, J.; Sun, J.; Sun, F.; Zhang, Y.; Zhang, C.; Du, Y. A New Hypervalent Iodine (Iii/v) Oxidant and Its Application to the Synthesis of 2H-Azirines. Chem. Sci. 2020, 11, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalali, M.; Bissember, A.C.; Yates, B.F.; Wengryniuk, S.E.; Ariafard, A. Oxidation of Electron-Deficient Phenols Mediated by Hypervalent Iodine(V) Reagents: Fundamental Mechanistic Features Revealed by a Density Functional Theory-Based Investigation. J. Org. Chem. 2021, 86, 12237–12246. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Hyun, S.M.; Wortman, A.K.; Powers, D.C. Oxidation Catalysis by an Aerobically Generated Dess-Martin Periodinane Analogue. Angew. Chem. 2018, 130, 7323–7327. [Google Scholar] [CrossRef]
- Pan, Y.; Li, J.; Li, Z.; Huang, F.; Ma, X.; Jiao, W.; Shao, H. An Efficient and Facile Strategy for Trifluoromethylation and Perfluoroalkylation of Isoquinolines and Heteroarenes. Chem. Commun. 2020, 56, 7813–7816. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sun, W.W.; Xie, Y.; Liu, J.K.; Liu, B.; Zhou, Y.; Wu, B. Metal-Free Regioselective Hypervalent Iodine-Mediated C-2 and C-3 Difunctionalization of N-Substituted Indoles. J. Org. Chem. 2016, 81, 11081–11094. [Google Scholar] [CrossRef]
- Bose, D.S.; Idrees, M. Dess-Martin Periodinane Mediated Intramolecular Cyclization of Phenolic Azomethines: A Solution-Phase Strategy toward Benzoxazoles and Benzothiazoles. Synthesis 2010, 3, 398–402. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Baran, P.S.; Zhong, Y.L.; Sugita, K. Iodine(V) Reagents in Organic Synthesis. Part 1. Synthesis of Polycyclic Heterocycles via Dess-Martin Periodinane-Mediated Cascade Cyclization: Generality, Scope, and Mechanism of the Reaction. J. Am. Chem. Soc. 2002, 124, 2212–2220. [Google Scholar] [CrossRef]
- Kupwade, R.V.; Khot, S.S.; Kulkarni, M.A.; Desai, U.V.; Wadgaonkar, P.P. Diethylamine Dess-Martin Periodinane: An Efficient Catalyst-Oxidant Combination in a Sequential, One-Pot Synthesis of Difficult to Access 2-Amino-3,5-Dicarbonitrile-6-Sulfanylpyridines at Ambient Temperature. RSC Adv. 2017, 7, 38877–38883. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.S.; Guo, R.N.; Cai, X.F.; Chen, M.W.; Shi, L.; Zhou, Y.G. Enantioselective Iridium-Catalyzed Hydrogenation of 1- and 3-Substituted Isoquinolinium Salts. Angew. Chem. 2013, 125, 3773–3777. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, L.; Yang, W.; Zhu, Y.; Fang, L.; Yu, C. Controllable Synthesis of 3-Chloro- and 3,3-Dichloro-2-Oxindoles via Hypervalent Iodine-Mediated Chlorooxidation. Org. Biomol. Chem. 2019, 17, 6920–6924. [Google Scholar] [CrossRef]
- Sun, W.W.; Chen, N.; Wei, T.T.; You, G.J.; Yang, H.; Liu, J.K.; Wu, B. Environmentally Friendly Protocol for 2,3-Difunctionlization of Indole Derivatives. J. Org. Chem. 2020, 85, 10143–10151. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Akamanchi, K.G. A Mild, Chemoselective, Oxidative Method for Deoximation Using Dess- Martin Periodinane. Synthesis 1999, 5, 760–764. [Google Scholar] [CrossRef]
- Nocquet, S.; Retailleau, P.; Cariou, K.; Dodd, R.H. Iodine (III)-Mediated Umpolung of Bromide Salts for the Ethoxybromination of Enamides. Org. Lett. 2013, 15, 1842–1845. [Google Scholar] [CrossRef] [PubMed]








 | |||
| Entry | Oxidant (Eq.) | Conditions | Yield (%) of 3aa/4 |
| 1 | DMP (2.0) | NMP, rt, air, 24 h | 62/trace |
| 2 | PIDA (2.0) | NMP, rt, air, 24 h | 0/0 |
| 3 | PIFA (2.0) | NMP, rt, air, 24 h | 0/0 |
| 4 | Koser’s reagent (2.0) | NMP, rt, air, 24 h | 0/0 |
| 5 | PhIO (2.0) | NMP, rt, air, 24 h | 0/0 |
| 6 | IBX (2.0) | NMP, rt, air, 24 h | 47/0 |
| 7 | DMP (2.0) | DMF, rt, air, 24 h | 38/0 |
| 8 | DMP (2.0) | MeCN, rt, air, 24 h | 55/0 |
| 9 | DMP (2.0) | DMSO, rt, air, 24 h | 0/0 |
| 10 | PIDA (2.0) | DMF, rt, air, 24 h | 0/37 |
| 11 | DMP (2.0) | NMP, −10 °C, air, 24 h | Trace/0 |
| 12 | DMP (2.0) | NMP, 0 °C, air, 72 h | 39/0 |
| 13 | DMP (2.0) | NMP, 50 °C, air, 24 h | 18/0 |
| 14 | DMP (1.0) | NMP, rt, air, 24 h | 21/0 |
| 15 | DMP (3.4) | NMP, rt, air, 36 h | 96/0 |
| 16 | DMP (3.4) | NMP, rt, N2, 36 h | 0/0 |
| 17 | DMP (3.4) | NMP, rt, air, H2O (10 eq.), 36 h | 54/0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhang, G.; Tang, S.; Pan, Y.; Shao, H.; Jiao, W. Dess–Martin Periodinane-Mediated Oxidative Coupling Reaction of Isoquinoline with Benzyl Bromide. Molecules 2023, 28, 923. https://doi.org/10.3390/molecules28030923
Yang C, Zhang G, Tang S, Pan Y, Shao H, Jiao W. Dess–Martin Periodinane-Mediated Oxidative Coupling Reaction of Isoquinoline with Benzyl Bromide. Molecules. 2023; 28(3):923. https://doi.org/10.3390/molecules28030923
Chicago/Turabian StyleYang, Chunmei, Guoqing Zhang, Senling Tang, Yang Pan, Huawu Shao, and Wei Jiao. 2023. "Dess–Martin Periodinane-Mediated Oxidative Coupling Reaction of Isoquinoline with Benzyl Bromide" Molecules 28, no. 3: 923. https://doi.org/10.3390/molecules28030923
APA StyleYang, C., Zhang, G., Tang, S., Pan, Y., Shao, H., & Jiao, W. (2023). Dess–Martin Periodinane-Mediated Oxidative Coupling Reaction of Isoquinoline with Benzyl Bromide. Molecules, 28(3), 923. https://doi.org/10.3390/molecules28030923