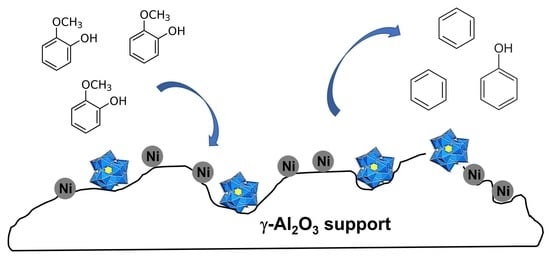
Heterogenization of Heteropolyacid with Metal-Based Alumina Supports for the Guaiacol Gas-Phase Hydrodeoxygenation
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
2.1.1. Structural Properties
2.1.2. Spectroscopic Properties
2.1.3. XPS Results
2.1.4. Acidity Properties
2.2. Catalytic Evaluation: HDO with Guaiacol
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ahmad, T.; Zhang, D. A Critical Review of Comparative Global Historical Energy Consumption and Future Demand: The Story Told so Far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Yıldız, İ. Fossil Fuels. In Comprehensive Energy Systems; Dincer, I.B.T.-C.E.S., Ed.; Elsevier: Oxford, UK, 2018; pp. 521–567. ISBN 978-0-12-814925-6. [Google Scholar]
- Zhong, J.; Pérez-Ramírez, J.; Yan, N. Biomass Valorisation over Polyoxometalate-Based Catalysts. Green Chem. 2021, 23, 18–36. [Google Scholar] [CrossRef]
- Attia, M.; Farag, S.; Chaouki, J. Upgrading of Oils from Biomass and Waste: Catalytic Hydrodeoxygenation. Catalysts 2020, 10, 1381. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Alsalme, A.; Alsharif, A.A.; Al-Enizi, H.; Khan, M.; Alshammari, S.G.; Alotaibi, M.A.; Khan, R.A.; Siddiqui, M.R.H. Probing the Catalytic Efficiency of Supported Heteropoly Acids for Esterification: Effect of Weak Catalyst Support Interactions. J. Chem. 2018, 2018, 7037461. [Google Scholar] [CrossRef] [Green Version]
- Marcilly, C. Acido-Basic Catalysis Application to Refining and Petrochemistry; Editions Technip: Paris, France, 2006; Volume 1, ISBN 2710808617. [Google Scholar]
- Song, W.; Liu, Y.; Baráth, E.; Zhao, C.; Lercher, J.A. Synergistic Effects of Ni and Acid Sites for Hydrogenation and C–O Bond Cleavage of Substituted Phenols. Green Chem. 2015, 17, 1204–1218. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Block, D.E.; Gates, B.C. Catalytic Conversion of Guaiacol Catalyzed by Platinum Supported on Alumina: Reaction Network Including Hydrodeoxygenation Reactions. Energy Fuels 2011, 25, 3417–3427. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Tahir, M.N. Advances in Graphene/Inorganic Nanoparticle Composites for Catalytic Applications. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Arumugam, P.; Govindasamy, G. SBA-15 and Carbon Supported Nickel, Heteropoly Acid Catalysts for the Hydrodeoxygenation of Lignin Derived Trans-Anethole to Sustainable Aviation Fuel. J. Porous Mater. 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Poole, O.; Alharbi, K.; Belic, D.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Hydrodeoxygenation of 3-Pentanone over Bifunctional Pt-Heteropoly Acid Catalyst in the Gas Phase: Enhancing Effect of Gold. Appl. Catal. B Environ. 2017, 202, 446–453. [Google Scholar] [CrossRef]
- Anderson, E.; Crisci, A.; Murugappan, K.; Román-Leshkov, Y. Bifunctional Molybdenum Polyoxometalates for the Combined Hydrodeoxygenation and Alkylation of Lignin-Derived Model Phenolics. ChemSusChem 2017, 10, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, X.; Yang, X.; Wang, X.; Chu, W.; Hu, Y. Heterogenization of Heteropolyacids: A General Discussion on the Preparation of Supported Acid Catalysts. Ind. Eng. Chem. Res. 1996, 35, 2546–2560. [Google Scholar] [CrossRef]
- Rafiee, E.; Eavani, S. Heterogenization of Heteropoly Compounds: A Review of Their Structure and Synthesis. RSC Adv. 2016, 6, 46433–46466. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Caliman, E.; Dias, J.A.; Dias, S.C.L.; Prado, A.G.S. Solvent Effect on the Preparation of H3PW12O40 Sup-ported on Alumina. Catal. Today 2005, 107–108, 816–825. [Google Scholar] [CrossRef]
- Liu, J.; Lei, J.; He, J.; Deng, L.; Wang, L.; Fan, K.; Rong, L. Hydroprocessing of Jatropha Oil for Production of Green Diesel over Non-Sulfided Ni-PTA/Al2O3 Catalyst. Sci. Rep. 2015, 5, 11327. [Google Scholar] [CrossRef] [Green Version]
- Forster, L.; Qie, Z.; Hu, M.; Mavridis, A.; Price, C.; Parlett, C.M.A.; Fan, X.; D’Agostino, C. Heteropolyacids Supported on Zirconia-Doped γ, θ and α Alumina: A Physicochemical Assessment and Characterisation of Supported Solid Acids. Appl. Surf. Sci. 2022, 605, 154696. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition Alumina Phases Induced by Heat Treatment of Boehmite: An X-ray Diffraction and Infrared Spectroscopy Study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Sasca, V.Z.; Verdes, O.; Avram, L.; Popa, A.; Erdőhelyi, A.; Oszko, A. The CsxH3−xPW12O40 Catalysts Microstructure Model. Appl. Catal. A Gen. 2013, 451, 50–57. [Google Scholar] [CrossRef]
- Sudhakar, P.; Pandurangan, A. Heteropolyacid (H3PW12O40)-Impregnated Mesoporous KIT-6 Catalyst for Green Synthesis of Bio-Diesel Using Transesterification of Non-Edible Neem Oil. Mater. Renew. Sustain. Energy 2019, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Naumkin, A.V.; Vass-Kraut, A.; Gaarensroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 10 February 2023).
- Basile, F.; Bergner, J.; Bombart, C.; Rondot, B.; Le Guevel, P.; Lorang, G. Electrochemical and Analytical (XPS and AES) Study of Passive Layers Formed on Fe–Ni Alloys in Borate Solutions. Surf. Interface Anal. 2000, 30, 154–157. [Google Scholar] [CrossRef]
- Nowińska, K.; Fiedorow, R.; Adamiec, J. Catalytic Activity of Supported Heteropoly Acids for Reactions Re-quiring Strong Acid Centres. J. Chem. Soc. Faraday Trans. 1991, 87, 749–775. [Google Scholar] [CrossRef]
- Boahene, P.E.; Soni, K.; Dalai, A.K.; Adjaye, J. Hydrotreating of Coker Light Gas Oil on Ti-Modified HMS Supports Using Ni/HPMo Catalysts. Appl Catal B Environ. 2011, 101, 294–305. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Wu, F.P.; Song, Q.L.; Fan, X.; Jin, L.J.; Wang, R.Y.; Cao, J.P.; Wei, X.Y. Hydrodeoxygenation of Lignin Model Compounds to Alkanes over Pd–Ni/HZSM-5 Catalysts. J. Energy Inst. 2020, 93, 899–910. [Google Scholar] [CrossRef]
- Itagaki, S.; Matsuhashi, N.; Taniguchi, K.; Yamaguchi, K.; Mizuno, N. Efficient Hydrodeoxygenation of Ketones, Phenols, and Ethers Promoted by Platinum-Heteropolyacid Bifunctional Catalysts. Chem. Lett. 2014, 43, 1086–1088. [Google Scholar] [CrossRef]
- Alharbi, K.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Hydrogenation of Ketones over Bifunctional Pt-Heteropoly Acid Catalyst in the Gas Phase. Appl. Catal. A Gen. 2015, 504, 457–462. [Google Scholar] [CrossRef]
- Popov, A.; Kondratieva, E.; Mariey, L.; Goupil, J.M.; El Fallah, J.; Gilson, J.P.; Travert, A.; Maugé, F. Bio-Oils Hydro-deoxygenation: Adsorption of Phenolic Molecules on Oxidic Catalyst Supports. J. Catal. 2010, 114, 176–186. [Google Scholar]
- Okuhara, T.; Watanabe, H.; Nishimura, T.; Inumaru, K.; Misono, M. Microstructure of Cesium Hydrogen Salts of 12-Tungstophosphoric Acid Relevant to Novel Acid Catalysis. Chem. Mater. 2000, 12, 2230–2238. [Google Scholar] [CrossRef]
- Kortum, G. Reflectance Spectroscopy: Principles, Methods, Applications; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar]














| Sample | Total Acidity (μmol·g−1) | LT Peak a (μmol·g−1) | HT Peak b (μmol·g−1) |
|---|---|---|---|
| Pt-Al2O3 | 223 | 134 | 89 |
| HPW/Pt-Al2O3 | 283 | 129 | 154 |
| Pt-Al2O3/Cs2.5 | 250 | 95 | 155 |
| Ni-Al2O3 | 352 | 239 | 113 |
| HPW/Ni-Al2O3 | 368 | 98 | 270 |
| Ni-Al2O3/Cs2.5 | 341 | 184 | 157 |
| Catalyst | Identified Compounds |
|---|---|
| Ni-Al2O3 | o-cresol, 1,2-dimethoxybenzene |
| HPW/Ni-Al2O3 | Cyclohexane, cyclohexene, toluene, o-cresol, 1-methoxy-3-methylbenzene |
| Ni-Al2O3/Cs2.5 | 1,2-dimethoxybenzene |
| Catalyst | Metal Content (wt.%) | HPW Content (wt.%) | Cs2.5 Content (wt.%) |
|---|---|---|---|
| Ni-Al2O3 | 28 | - | - |
| HPW/Ni-Al2O3 | 18 | - | |
| Ni-Al2O3/Cs2.5 | - | 20 | |
| Pt-Al2O3 | 1 | - | - |
| HPW/Pt-Al2O3 | 18 | - | |
| Pt-Al2O3/Cs2.5 | - | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, R.F.; Costa, D.; Ferraria, A.M.; Botelho do Rego, A.M.; Ribeiro, F.; Martins, Â.; Fernandes, A. Heterogenization of Heteropolyacid with Metal-Based Alumina Supports for the Guaiacol Gas-Phase Hydrodeoxygenation. Molecules 2023, 28, 2245. https://doi.org/10.3390/molecules28052245
Nunes RF, Costa D, Ferraria AM, Botelho do Rego AM, Ribeiro F, Martins Â, Fernandes A. Heterogenization of Heteropolyacid with Metal-Based Alumina Supports for the Guaiacol Gas-Phase Hydrodeoxygenation. Molecules. 2023; 28(5):2245. https://doi.org/10.3390/molecules28052245
Chicago/Turabian StyleNunes, Rita F., Daniel Costa, Ana M. Ferraria, Ana M. Botelho do Rego, Filipa Ribeiro, Ângela Martins, and Auguste Fernandes. 2023. "Heterogenization of Heteropolyacid with Metal-Based Alumina Supports for the Guaiacol Gas-Phase Hydrodeoxygenation" Molecules 28, no. 5: 2245. https://doi.org/10.3390/molecules28052245
APA StyleNunes, R. F., Costa, D., Ferraria, A. M., Botelho do Rego, A. M., Ribeiro, F., Martins, Â., & Fernandes, A. (2023). Heterogenization of Heteropolyacid with Metal-Based Alumina Supports for the Guaiacol Gas-Phase Hydrodeoxygenation. Molecules, 28(5), 2245. https://doi.org/10.3390/molecules28052245