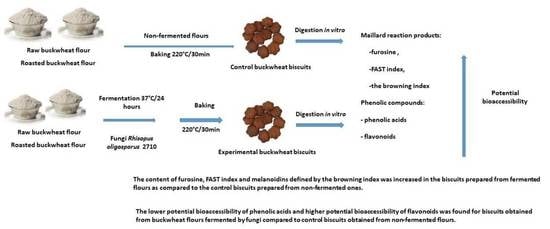
Identification and Bioaccessibility of Maillard Reaction Products and Phenolic Compounds in Buckwheat Biscuits Formulated from Flour Fermented by Rhizopus oligosporus 2710
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Maillard Reaction Indexes and Their Bioaccessibility from Investigated Samples
2.2. Phenolic Acids and Their Potential Bioaccessibility from Investigated Samples
2.3. Flavonoids and Their Potential Bioaccessibility from Investigated Samples
3. Experimental
3.1. Chemicals
3.2. Buckwheat Flour
3.3. Pretreatment of Buckwheat Flour
3.4. Fermentation of Buckwheat Flours by Rhizopus oligosporus 2710, Preparation of Buckwheat Biscuits from Fermented Flours, and In Vitro Digestion
3.4.1. Fermentation of Buckwheat Flour
3.4.2. Preparation of Buckwheat Biscuits from Fermented Flour
3.4.3. In Vitro Digestion of Buckwheat Biscuits
3.5. Maillard Reaction Products Determination
3.6. Extraction and Isolation of the Main Phenolic Compounds and Flavonoids from Analyzed Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Astuti, M.; Meliala, A.; Dalais, F.S.; Wahlqvist, M.L. Tempe, a nutritious and healthy food from Indonesia. Asia Pac. J. Clin. Nutr. 2000, 9, 322–325. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez- Avila, G.; Montañez- Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid- state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, C.; Li, Y.; Zhang, Y.; Liu, L.; Wu, Z.; Weng, P. Insights into oat polyphenols constituent against advanced glycation end products mechanism by spectroscopy and molecular interaction. Food Biosci. 2021, 43, 101313. [Google Scholar] [CrossRef]
- Randhir, R.; Wattem, D.; Shetty, K. Solid-state bioconversion of fava bean by Rhizopus oligosporus for enrichment of phenolic anti-oxidants and L-DOPA. Int. J. Food Sci. Technol. 2004, 5, 235–244. [Google Scholar]
- Sheih, I.-C.; Wu, H.-Y.; Lai, Y.-J.; Lin, C.-F. Preparation of high free radical scavenging tempeh by newly isolated Rhizopus sp. R-69 from Indonesia. Food Sci. Agric. Chem. 2000, 10, 35–40. [Google Scholar]
- Wronkowska, M.; Christa, K.; Ciska, E.; Soral-Śmietana, M. Chemical characteristics and sensory evaluation of raw and roasted buckwheat groats fermented by Rhizopus oligosporus. J. Food Qual. 2015, 38, 130–138. [Google Scholar] [CrossRef]
- Wronkowska, M.; Honke, J.; Piskuła, M.K. Effect of solid-state fermentation with Rhizopus oligosporus on bioactive compounds and antioxidant capacity of raw and roasted buckwheat groats. Ital. J. Food Sci. 2015, 27, 424–431. [Google Scholar]
- Zielińska, D.; Szawara-Nowak, D.; Michalska, A. Antioxidant capacity of thermally-treated buckwheat. Pol. J. Food Nutr. Sci. 2007, 57, 465–470. [Google Scholar]
- Zieliński, H.; Honke, J.; Bączek, N.; Majkowska, A.; Wronkowska, M. Bioaccessibility of D-chiro-inositol from water biscuits formulated from buckwheat flours fermented by lactic acid bacteria and fungi. LWT Food Sci. Technol. 2019, 106, 37–43. [Google Scholar] [CrossRef]
- Zieliński, H.; Wiczkowski, W.; Honke, J.; Piskuła, M.K. In Vitro Expanded Bioaccessibility of Quercetin-3-Rutinoside and Quercetin Aglycone from Buckwheat Biscuits Formulated from Flours Fermented by Lactic Acid Bacteria. Antioxidants 2021, 10, 571. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, F. Simulating human gastrointestinal motility in dynamic in vitro models. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3804–3833. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.; Morales, F.J. Study on fluorescence of Maillard reaction compounds in breakfast cereals. Mol. Nutr. Food Res. 2006, 50, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Zielińska, D.; Kostyra, H. Antioxidant capacity of a new crispy type food product determined by updated analytical strategies. Food Chem. 2012, 130, 1098–1104. [Google Scholar] [CrossRef]
- Damjanovic Desic, S.; Birlouez-Aragon, I. The FAST index—A highly sensitive indicator of the heat impact on infant formula model. Food Chem. 2011, 124, 1043–1049. [Google Scholar] [CrossRef]
- Zieliński, H.; Michalska, A.; Amigo-Benavent, M.; del Castillo, M.D.; Piskuła, M.K. Changes in Protein Quality and Antioxidant Properties of Buckwheat Seeds and Groats Induced by Roasting. J. Agric. Food Chem. 2009, 57, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Wronkowska, M.; Jeliński, T.; Majkowska, A.; Zieliński, H. Physical properties of buckwheat water biscuits formulated on fermented flours by selected lactic acid bacteria. Pol. J. Food Nutr. Sci. 2018, 68, 25–31. [Google Scholar] [CrossRef]
- Tekliye, M.; Pei, X.; Dong, M. RP-HPLC determination of Furosine in fermented milk of different brands retailed in China. Int. J. Agric. Sci. Food Technol. 2019, 5, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Yıltırak, S.; Kocadağli, T.; Çelik, E.E.; Kanmaz, E.O.; Gökmen, V. Effects of sprouting and fermentation on the formation of Maillard reaction products in different cereals heated as wholemeal. Food Chem. 2022, 389, 133075. [Google Scholar] [CrossRef]
- Zieliński, H.; Ciesarová, Z.; Kukurová, K.; Zielińska, D.; Szawara-Nowak, D.; Starowicz, M.; Wronkowska, M. Effect of fermented and unfermented buckwheat flour on functional properties of gluten-free muffins. J. Food Sci. Technol. 2017, 54, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of nonenzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2001, 11, 340–346. [Google Scholar] [CrossRef]
- Zieliński, H.; Szawara-Nowak, D.; Bączek, N.; Wronkowska, M. Effect of liquid-state fermentation on the antioxidant and functional properties of raw and roasted buckwheat flours. Food Chem. 2019, 271, 291–297. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Olech, M. The Impact of Processing Parameters on the Content of Phenolic Compounds in New Gluten-Free Precooked Buckwheat Pasta. Molecules 2019, 24, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of Free and Bound Phenolic Compounds in Buckwheat Spaghetti by RP-HPLC-ESI-TOF-MS: Effect of Thermal Processing from Farm to Fork. J. Agric. Food Chem. 2011, 59, 7700–7707. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Duliński, R.; Stodolak, B.; Mickowska, B.; Wikiera, A. Prolonged tempe-type fermentation in order to improve bioactive potential and nutritional parameters of quinoa seeds. J. Cereal Sci. 2016, 71, 116–121. [Google Scholar] [CrossRef]
- Choi, A.S.; Bea, I.Y.; Lee, H.G. Predicting buckwheat flavonoids bioavailability in different food matrices under in vitro simulated human digestion. Cereal Chem. 2017, 94, 310–314. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. AACC Official Methods 10-52, Baking Quality of Cookie Flour – Micro Method. Approved Methods of the American Association of Cereal Chemists, 9th ed.; AACC: Minneapolis, MN, USA, 1995. [Google Scholar]
- Hidalgo, A.; Brandolini, A. Heat damage of water biscuits from einkorn, durum and bread wheat flours. Food Chem. 2011, 128, 471–478. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Conde-Aguilera, J.A.; Haro, A.; De La Cueva, S.P.; Rufián Henares, J.A. A combined procedure to evaluate the global antioxidant response of bread. J. Cereal Sci. 2010, 56, 239–246. [Google Scholar]
- Wronkowska, M.; Piskuła, M.K.; Zieliński, H. Effect of roasting time of buckwheat groats on the formation of Maillard reaction products and antioxidant capacity. Food Chem. 2016, 196, 355–358. [Google Scholar]
- Jeż, M.; Wiczkowski, W.; Zielińska, D.; Białobrzeski, I.; Błaszczak, W. The impact of high pressure processing on the phenolic profile, hydrophilic antioxidant and reducing capacity of purée obtained from commercial tomato varieties. Food Chem. 2018, 261, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Płatosz, N.; Sawicki, T.; Wiczkowski, W. Profile of phenolic acids and flavonoids of red beet and its fermentation products. Does long-term consumption of fermented beetroot juice affect phenolics profile in human blood plasma and urine? Pol. J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]


| Sample | Maillard Reaction Products | Biscuits | Digested Biscuits | PB |
|---|---|---|---|---|
| Raw | ||||
| Control | Furosine (mg/g DM) | 3.28 ± 0.04 bB | 7.53 ± 0.51 bA | 2.3 |
| Fermented | 20.27 ± 0.07 aB | 41.09 ± 2.74 aA | 2.0 | |
| Control | FAST index (%) | 262.2 ± 18.4 bA | 55.7 ± 2.1 bB | 0.2 |
| Fermented | 504.8 ± 40.4 aA | 203.0 ± 23.5 aB | 0.4 | |
| Control | Soluble proteins (mg/g DM) | 41.05 ± 0.23 bB * | 99.03 ± 5.47 bA | 2.4 |
| Fermented | 115.14 ± 0.42 aB | 225.29 ± 16.46 aA | 1.9 | |
| Control | Browning index (AU) | 0.21 ± 0.01 bB | 0.96 ± 0.02 bA | 4.6 |
| Fermented | 1.05 ± 0.19 aB | 3.34 ± 0.23 aA | 3.2 | |
| Roasted | ||||
| Control | Furosine (mg/g DM) | 1.06 ± 0.04 bB | 5.20 ± 0.83 bA | 4.9 |
| Fermented | 18.41 ± 0.06 aB | 35.31 ± 2.55 aA | 1.9 | |
| Control | FAST index (%) | 214.0 ± 4.6 bA | 55.0 ± 0.5 bB | 0.3 |
| Fermented | 445.3 ± 9.3 aA | 117.0 ± 6.4 aB | 0.3 | |
| Control | Soluble proteins (mg/g DM) | 29.26 ± 0.17 bB | 68.53 ± 3.80 bA | 2.3 |
| Fermented | 62.68 ± 0.89 aB | 170.09 ± 3.23 aA | 2.7 | |
| Control | Browning index (AU) | 0.32 ± 0.01 bB | 1.09 ± 0.04 aA | 3.4 |
| Fermented | 0.50 ± 0.05 aB | 1.97 ± 0.07 aA | 3.9 | |
| Sample | Phenolic Acids | Flour | Biscuits | Digested Biscuits | PB |
|---|---|---|---|---|---|
| Raw | |||||
| Control | Ferulic | 122.67 ± 4.12 aA | 3.21 ± 0.13 bC | 8.17 ± 0.21 aB | 2.5 |
| Fermented | 78.05 ± 2.08 bA | 5.98 ± 0.16 aB | 5.22 ± 0.14 bB | 0.9 | |
| Control | Syringic | 78.80 ± 0.85 aB | 43.63 ± 1.33 aC | 130.65 ± 1.22 aA | 3.0 |
| Fermented | 41.82 ± 1.60 bB | 37.16 ± 1.12 bB | 88.06 ± 1.80 bA | 2.4 | |
| Control | Vanillic | 55.52 ± 2.08 bC | 112.66 ± 2.66 aB | 187.90 ± 18.83 aA | 1.7 |
| Fermented | 94.83 ± 2.55 aAB | 80.12 ± 2.68 bB | 114.94 ± 4.87 bA | 1.4 | |
| Control | Protocatechuic | 26.57 ± 0.78 aC | 65.79 ± 2.46 aB | 203.57 ± 6.15 aA | 3.1 |
| Fermented | 20.44 ± 0.68 aB | 27.40 ± 1.28 bB | 124.06 ± 0.71 bA | 4.5 | |
| Control | p-Coumaric | 22.13 ± 0.24 aA | 21.53 ± 3.10 bA | 26.33 ± 0.14 aA | 1.2 |
| Fermented | 13.72 ± 0.57 bC | 32.66 ± 0.44 aA | 22.80 ± 0.42 aB | 0.7 | |
| Control | Caffeic | 21.80 ± 0.08 bA | 3.40 ± 0.11 bC | 14.64 ± 0.09 bB | 4.3 |
| Fermented | 46.88 ± 0.98 aA | 12.47 ± 0.38 aC | 21.15 ± 0.36 aB | 1.7 | |
| Control | t-Cinnamic | 7.08 ± 0.10 aB | 7.86 ± 0.02 aB | 8.29 ± 0.02 aA | 1.1 |
| Fermented | 0.33 ± 0.02 bC | 2.82 ± 0.08 bB | 4.84 ± 0.16 bA | 1.7 | |
| Control | Sinapic | 6.00 ± 0.33 bB | 8.33 ± 0.10 bB | 22.16 ± 0.30 aA | 2.7 |
| Fermented | 9.14 ± 0.27 aB | 17.84 ± 1.42 aA | 16.90 ± 1.54 bA | 0.9 | |
| Control | Chlorogenic | 0.04 ± 0.00 bA | 0.08 ± 0.00 aA | 0.23 ± 0.10 aA | 2.9 |
| Fermented | 0.27 ± 0.00 aA | 0.09 ± 0.00 aA | 0.09 ± 0.00 bA | 1.0 | |
| Control | Isovanillic | n.d. | 2.10 ± 0.05 bB | 17.41 ± 0.37 aA | 8.3 |
| Fermented | n.d. | 5.96 ± 0.34 aB | 17.93 ± 0.09 aA | 3.0 | |
| Roasted | |||||
| Control | Ferulic | 23.50 ± 2.43 bA | 2.55 ± 0.07 aC | 4.93 ± 0.12 bB | 1.9 |
| Fermented | 30.55 ± 3.00 aA | 2.52 ± 0.06 aC | 7.49 ± 0.19 aB | 3.0 | |
| Control | Syringic | 24.66 ± 0.74 bC | 100.93 ± 2.05 aA | 80.86 ± 4.29 bB | 0.8 |
| Fermented | 86.90 ± 1.60 aB | 11.55 ± 0.31 bC | 94.21 ± 2.49 aA | 8.2 | |
| Control | Vanillic | 68.62 ± 1.07 bB | 49.74 ± 2.19 aC | 111.25 ± 4.11 bA | 2.2 |
| Fermented | 187.76 ± 6.25 aA | 31.41 ± 0.90 bC | 123.68 ± 2.70 aB | 3.9 | |
| Control | Protocatechuic | 25.24 ± 0.32 bB | 29.96 ± 0.57 aB | 151.53 ± 4.45 bA | 5.1 |
| Fermented | 31.28 ± 0.57 aB | 25.03 ± 0.70 bC | 188.21 ± 2.67 aA | 7.5 | |
| Control | p-Coumaric | 13.52 ± 0.13 bA | 7.53 ± 0.34 bB | 14.94 ± 0.17 bA | 2.0 |
| Fermented | 31.00 ± 2.13 aA | 13.28 ± 0.10 aC | 18.46 ± 0.22 aB | 1.4 | |
| Control | Caffeic | 89.33 ± 3.82 aA | 0.70 ± 0.00 bC | 29.13 ± 0.79 bB | 41.6 |
| Fermented | 70.15 ± 0.56 bA | 6.04 ± 0.17 aC | 35.85 ± 0.51 aB | 5.9 | |
| Control | t-Cinnamic | 9.98 ± 0.19 aA | 2.52 ± 0.05 aC | 6.32 ± 0.06 bB | 2.5 |
| Fermented | 0.54 ± 0.03 bC | 1.60 ± 0.06 bB | 7.80 ± 0.06 aA | 4.9 | |
| Control | Sinapic | 8.19 ± 0.55 aB | 1.97 ± 0.03 bC | 12.07 ± 0.30 bA | 6.1 |
| Fermented | 6.14 ± 0.07 bB | 3.60 ± 0.12 aC | 13.99 ± 0.40 aA | 3.9 | |
| Control | Chlorogenic | 0.07 ± 0.00 aA | 0.06 ± 0.00 aA | 0.11 ± 0.00 aA | 1.8 |
| Fermented | 0.17 ± 0.00 aA | 0.10 ± 0.00 aA | 0.11 ± 0.00 aA | 1.1 | |
| Control | Isovanillic | n.d. | 3.12 ± 0.17 bB | 19.09 ± 3.74 aA | 6.1 |
| Fermented | n.d. | 4.87 ± 0.23 aB | 14.48 ± 0.29 bA | 3.0 | |
| Sample | Flavonoids | Flour | Biscuits | Digested Biscuits | PB |
|---|---|---|---|---|---|
| Raw | |||||
| Control | Rutin | 376.40 ± 6.30 aA * | 90.53 ± 3.45 bB * | 1.90 ± 0.07 bC * | 0.02 |
| Fermented | 367.80 ± 1.80 aA | 150.77 ± 5.09 aB | 2.23 ± 0.04 aC | 0.01 | |
| Control | Epicatechin | 183.33 ± 0.64 aA | 91.69 ± 2.73 aB | 16.45 ± 0.53 aC | 0.2 |
| Fermented | 64.34 ± 0.08 bA | 2.39 ± 0.07 bC | 10.40 ± 0.24 bB | 4.4 | |
| Control | Vitexin | 21.24 ± 1.00 aA | 15.04 ± 0.21 aB | 8.30 ± 0.29 aC | 0.6 |
| Fermented | 10.29 ± 0.58 bB | 13.51 ± 0.35 bA | 6.58 ± 0.19 bC | 0.5 | |
| Control | Orientin | 17.62 ± 0.25 bA | 4.21 ± 0.18 aB | 4.23 ± 0.04 aB | 1.0 |
| Fermented | 20.08 ± 0.16 aA | 4.47 ± 0.18 aBC | 2.23 ± 0.05 bC | 0.5 | |
| Control | Quercitin | 8.32 ± 0.02 aA * | 4.55 ± 0.25 aC * | 7.55 ± 0.28 aB * | 1.7 |
| Fermented | 3.80 ± 0.01 bB | 1.41 ± 0.06 bC | 6.77 ± 0.20 bA | 4.8 | |
| Control | Kaempferol | 1.12 ± 0.02 aB | 0.75 ± 0.12 aC | 9.27 ± 0.08 bA | 12.4 |
| Fermented | 0.31 ± 0.07 bB | 0.35 ± 0.09 bB | 10.34 ± 0.26 aA | 29.5 | |
| Control | Apigenin | 0.30 ± 0.04 bC | 2.13 ± 0.20 aA | 1.25 ± 0.02 aB | 0.6 |
| Fermented | 2.35 ± 0.02 aA | 0.92 ± 0.03 bC | 1.24 ± 0.01 aB | 1.3 | |
| Control | Luteolin | 0.26 ± 0.02 aA | 0.22 ± 0.02 aA | 0.19 ± 0.02 aA | 0.9 |
| Fermented | 0.09 ± 0.01 bB | 0.11 ± 0.04 bA | 0.18 ± 0.08 aA | 1.6 | |
| Roasted | |||||
| Control | Rutin | 220.40 ± 0.30 * | 61.00 ± 3.04 * | 2.51 ± 0.10 * | 0.04 |
| Fermented | 150.60 ± 6.90 | 92.60 ± 6.71 | 5.19 ± 0.04 | 0.06 | |
| Control | Epicatechin | 138.48 ± 0.77 aA | 6.25 ± 0.20 aC | 18.22 ± 0.51 aB | 2.9 |
| Fermented | 86.85 ± 0.36 bA | 2.18 ± 0.10 bC | 18.74 ± 0.43 aB | 8.6 | |
| Control | Vitexin | 22.41 ± 1.22 aA | 10.26 ± 0.04 aB | 7.56 ± 0.15 aC | 0.7 |
| Fermented | 11.31 ± 0.61 bA | 9.23 ± 0.17 aB | 7.75 ± 0.20 aC | 0.8 | |
| Control | Orientin | 14.19 ± 0.45 aA | 5.29 ± 0.01 aB | 3.31 ± 0.06 aB | 0.6 |
| Fermented | 8.04 ± 0.40 bA | 1.54 ± 0.05 bC | 2.88 ± 0.08 aB | 1.9 | |
| Control | Quercitin | 3.82 ± 0.04 aB * | 1.37 ± 0.01 aC * | 7.72 ± 0.45 bA * | 5.6 |
| Fermented | 2.80 ± 0.01 bB | 0.29 ± 0.01 bC | 11.88 ± 0.19 aA | 41.0 | |
| Control | Kaempferol | 0.78 ± 0.01 aC | 3.39 ± 0.27 aB | 17.26 ± 0.54 bA | 5.1 |
| Fermented | 0.40 ± 0.02 bC | 1.41 ± 0.21 bB | 18.87 ± 0.42 aA | 13.4 | |
| Control | Apigenin | 0.71 ± 0.00 bC | 1.54 ± 0.05 aA | 1.16 ± 0.02 aB | 0.8 |
| Fermented | 0.95 ± 0.02 aB | 1.08 ± 0.02 bB | 1.16 ± 0.02 aA | 1.1 | |
| Control | Luteolin | 0.24 ± 0.01 aB | 0.15 ± 0.03 aC | 0.38 ± 0.03 aA | 2.5 |
| Fermented | 0.08 ± 0.01 bC | 0.15 ± 0.01 aB | 0.38 ± 0.01 aA | 2.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wronkowska, M.; Wiczkowski, W.; Topolska, J.; Szawara-Nowak, D.; Piskuła, M.K.; Zieliński, H. Identification and Bioaccessibility of Maillard Reaction Products and Phenolic Compounds in Buckwheat Biscuits Formulated from Flour Fermented by Rhizopus oligosporus 2710. Molecules 2023, 28, 2746. https://doi.org/10.3390/molecules28062746
Wronkowska M, Wiczkowski W, Topolska J, Szawara-Nowak D, Piskuła MK, Zieliński H. Identification and Bioaccessibility of Maillard Reaction Products and Phenolic Compounds in Buckwheat Biscuits Formulated from Flour Fermented by Rhizopus oligosporus 2710. Molecules. 2023; 28(6):2746. https://doi.org/10.3390/molecules28062746
Chicago/Turabian StyleWronkowska, Małgorzata, Wiesław Wiczkowski, Joanna Topolska, Dorota Szawara-Nowak, Mariusz Konrad Piskuła, and Henryk Zieliński. 2023. "Identification and Bioaccessibility of Maillard Reaction Products and Phenolic Compounds in Buckwheat Biscuits Formulated from Flour Fermented by Rhizopus oligosporus 2710" Molecules 28, no. 6: 2746. https://doi.org/10.3390/molecules28062746
APA StyleWronkowska, M., Wiczkowski, W., Topolska, J., Szawara-Nowak, D., Piskuła, M. K., & Zieliński, H. (2023). Identification and Bioaccessibility of Maillard Reaction Products and Phenolic Compounds in Buckwheat Biscuits Formulated from Flour Fermented by Rhizopus oligosporus 2710. Molecules, 28(6), 2746. https://doi.org/10.3390/molecules28062746