Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities
Abstract
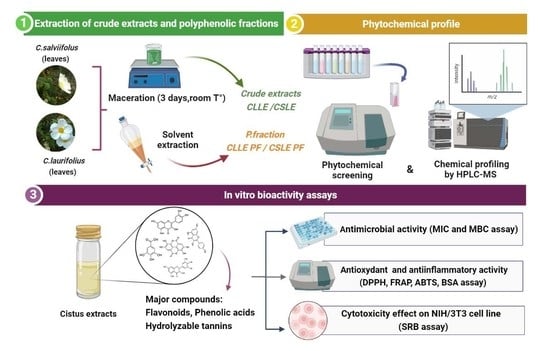
:1. Introduction
2. Results
2.1. Phytochemicals
2.1.1. Colorimetric Analysis
2.1.2. HPLC-DAD and HPLC-ESI-/MS Based Secondary Metabolic Profiles
2.2. Antioxidant Activity
2.3. Anti-Inflammatory Activity
2.4. Antimicrobial Activity
2.5. Cytotoxicity Evaluation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of the Extracts
4.3. Chemical Characterization
4.3.1. Phytochemical Screening
Total Polyphenol Content
Flavonoids Content
Flavonols Content
4.3.2. HPLC-DAD and HPLC-ESI-/MS Based Secondary Metabolic Profiles
4.4. Antioxidant Activity
4.5. Anti-Inflammatory Activity
4.6. Antimicrobial Activity
4.6.1. Microbial Strains and Culture Conditions
4.6.2. MIC and MBC/MFC Determination
4.7. Cytotoxicity Evaluation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, H.; Bahmani, M.; Shahinfard, N.; Nafchi, A.M.; Saberianpour, S.; Kopaei, M.R. Medicinal plants for the treatment of acne vulgaris: A review of recent evidences. Jundishapur J. Microbiol. 2015, 8, e25580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayel, K.; Attia, M.; Agamia, N.; Fadl, N. Acne vulgaris: Prevalence, severity, and impact on quality of life and self-esteem among Egyptian adolescents. J. Egypt Public Health Assoc. 2020, 95, 30. [Google Scholar] [CrossRef] [PubMed]
- Heng, A.H.; Chew, F.T. Systematic review of the epidemiology of acne vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degitz, K.; Placzek, M.; Borelli, C.; Plewig, G. Pathophysiology of acne. JDDG 2007, 5, 316–323. [Google Scholar] [CrossRef]
- Webster, G. Mechanism-based treatment of acne vulgaris: The value of combination therapy. J. Drugs Dermatol. 2005, 4, 281–288. [Google Scholar]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of acne vulgaris: A review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Del Rosso, J.Q.; Mancini, A.J.; Cook-Bolden, F.; Desai, S.; Weiss, J.; Pariser, D.; Zeichner, J.; Bhatia, N.; Kircik, L. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J. Drugs Dermatol. 2015, 14, 263–272. [Google Scholar]
- VandenBergh, B.; Michiels, J.E.; Wenseleers, T.; Windels, E.M.; Boer, P.V.; Kestemont, D.; De Meester, L.; Verstrepen, K.J.; Verstraeten, N.; Fauvart, M.; et al. Frequency of antibiotic application drives rapid evolutionary adaptation of Escherichia coli persistence. Nat. Microbiol. 2016, 1, 16020. [Google Scholar] [CrossRef] [Green Version]
- Layton, A. The use of isotretinoin in acne. Derm.-Endocrinol. 2009, 1, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Alaoui-Faris, E.; Mrabet, N.; Tahiri, H. Nombre chromosomique et caryotype de Cistus ladanifer subsp. Africanus dansereau (Cistaceae). Lagascalia 2009, 29, 23–27. [Google Scholar]
- Attaguile, G.; Perticone, G.; Mania, G.; Savoca, F.; Pennisi, G.; Salomone, S. Cistus incanus and Cistus monspeliensis inhibit the contractile response in isolated rat smooth muscle. J. Ethnopharmacol. 2004, 92, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Saracini, E.; Tattini, M.; Traversi, M.L.; Vincieri, F.F.; Pinelli, P. Simultaneous LC-DAD and LC-MS determination of ellagitannins, flavonoid glycosides, and acyl-glycosyl flavonoids in Cistus salvifolius L. leaves. Chromatographia. 2005, 62, 245–249. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical composition, biological and cytotoxic activities of Cistus salviifolius flower buds and leaves extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Jemia, M.B.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sarici, G.; Cinar, S.; Armutcu, F.; Altınyazar, C.; Koca, R.; Tekin, N.S. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 7637. [Google Scholar] [CrossRef]
- Puglia, C.; Santagati, N.A.; Bonina, F.; Trombetta, D.; Cristani, M.; Speciale, A.; Saija, A. Protective effect of Mediterranean fish oil extracts on heat-induced denaturation of albumin. J. Pharm. Pharmacol. 2006, 58, 1411–1413. [Google Scholar] [CrossRef]
- Levison, M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Orhan, N.; Aslan, M.; Şüküroğlu, M.; Orhan, D.D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLC–TOF-MS analysis. J. Ethnopharmacol. 2013, 146, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Włodarczyk, M.; Starzec, A. Isolation and structure elucidation of cistusin: A new ellagitannin from Cistus× incanus L. leaves. Ind. Crops Prod. 2020, 158, 112971. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Lee, H.; Lee, C.S. Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κB pathways in human keratinocytes. Eur. J. Pharmacol. 2016, 784, 164–172. [Google Scholar] [CrossRef]
- Srimathi Priyanga, K.; Vijayalakshmi, K. Investigation of antioxidant potential of quercetin and hesperidin: An in vitro approach. Asian J. Pharm. Clin. Res. 2017, 10, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- de Melo, L.F.M.; Aquino-Martins, V.G.Q.; daSilva, A.P.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Güvenç, A.; Yıldız, S.; Özkan, A.M.; Erdurak, C.S.; Coşkun, M.; Yılmaz, G.; Okuyama, T.; Okada, T. Antimicrobiological studies on turkish Cistus species. Pharm. Biol. 2005, 43, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Zeouk, I.; Balouiri, M.; Bekhti, K. Antistaphylococcal activity and phytochemical analysis of crude extracts of five medicinal plants used in the center of Morocco against dermatitis. Int. J. Microbiol. 2019, 4, 1803102. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their poly-phenolic composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Berrin-Ozcelik, O.U.; Baykal, T. Bioactivities of ethanolic extract and its fractions of Cistus laurifolius L. (Cistaceae) and Salvia wiedemannii Boiss. (Lamiaceae) species. Pharmacogn. Mag. 2016, 12, 82. [Google Scholar]
- Nur Onal, F.; Ozturk, I.; Aydin Kose, F.; Der, G.; Kilinc, E.; Baykan, S. Comparative evaluation of polyphenol contents and biological activities of five Cistus L. species native to Turkey. Chem. Biodivers. 2023, 20, e202200915. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Bouaziz, M.; Jemai, H.; Khabou, W.; Sayadi, S. Oil content, phenolic profiling and antioxidant potential of Tunisian olive drupes. J. Sci. Food Agric. 2010, 90, 1750–1758. [Google Scholar]
- Russo, D.; Miglionico, R.; Carmosino, M.; Bisaccia, F.; Andrade, P.B.; Valentão, P.; Milella, L.; Armentano, M.F. A comparative study on phytochemical profiles and biological activities of Sclerocarya birrea (A. Rich.) Hochst leaf and bark extracts. Int. J. Mol. Sci. 2018, 19, 186. [Google Scholar] [CrossRef] [Green Version]
- Abidi, J.; Occhiuto, C.; Cimino, F.; Speciale, A.; Ruberto, G.; Siracusa, L.; Bouaziz, M.; Boumendjel, M.; Muscarà, C.; Saija, A.; et al. Phytochemical and biological characterization of methanolic extracts from Rumex algeriensis and Rumex tunetanus. Chem. Biodivers. 2020, 17, e2000345. [Google Scholar] [PubMed]
- Boussahel, S.; Speciale, A.; Dahamna, S.; Amar, Y.; Bonaccorsi, I.; Cacciola, F.; Cimino, F.; Donato, P.; Ferlazzo, G.; Harzallah, D.; et al. Flavonoid profile, antioxidant and cytotoxic activity of different extracts from Algerian Rhamnus alaternus L. bark. Pharmacogn. Mag. 2015, 11, S102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard, 11th ed. Clinical and Laboratory Standards Institute (CLSI): Wayne, IL, USA, 2018.
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard. Clinical and Laboratory Standards Institute (CLSI): Wayne, IL, USA, 2008.
- Chelly, S.; Chelly, M.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Molonia, M.S.; Ruberto, G.; D’Angelo, V.; Germanò, M.P.; et al. Evaluation of antioxidant, anti-Inflammatory and antityrosinase potential of extracts from different aerial parts of Rhanterium suaveolens from Tunisia. Chem. Biodivers. 2021, 18, e2100316. [Google Scholar] [CrossRef] [PubMed]


| Crude Extracts | Polyphenol Fractions | |||
|---|---|---|---|---|
| CLLE | CSLE | CLLE PF | CSLE PF | |
| Yield % | 15.8 ± 0.8 | 15.2 ± 1.0 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Total polyphenol content mgGAE/g DE | 110.2 ± 59.7 | 258.4 ± 28.2 | 419.8 ± 9.2 | 456.1 ± 24.4 |
| Flavonoids mgCatE/g DE | 113.7 ± 32.4 | 111.9 ± 11.8 | 172.2 ± 26.6 | 144.4 ± 10.3 |
| Flavonols mgQE/g DE | 56.2 ± 5.2 | 33.9 ± 0.5 | 123.4 ± 6.4 | 114.7 ± 14.4 |
| Peak | Compound | CLLE | CSLE | CLLE PF | CSLE PF |
|---|---|---|---|---|---|
| 1 | P-coumaroyl quinic acid, isomer 1 | 0.0337 | n.d. * | n.d. | n.d. |
| 2 | Terflavin A anomer 1 | n.d. | 0.6234 | n.d. | 0.2343 |
| 3 | P-coumaroyl quinic acid, isomer 2 | 0.0385 | n.d. | n.d. | n.d. |
| 4 | Cistusin anomer 1 | n.d. | 0.0732 | n.d. | 0.3984 |
| 5 | Gallagic acid derivative | n.d. | 0.5044 | n.d. | 0.5476 |
| 6 | P-coumaroyl glucose, isomer 1 | 0.0560 | n.d. | 0.0403 | n.d. |
| 7 | Terflavin A anomer 2 | n.d. | 0.0537 | n.d. | 0.0271 |
| 8 | P-coumaroyl glucose, isomer 2 | 0.0864 | n.d. | 0.0641 | n.d. |
| 9 | Cistusin anomer 2 | n.d. | 0.1247 | n.d. | 0.2652 |
| 10 | Myricetin hexoside derivative | 1.4631 | 0.2132 | 8.7267 | 2.6069 |
| 11 | Quercetin derivative | 0.0534 | 0.0114 | 0.3354 | 0.1971 |
| 12 | Feruloyl glucose | 0.0540 | 0.0253 | 0.4464 | n.d. |
| 13 | Myricetin hexoside | 0.2351 | 0.1822 | 2.5111 | 2.9201 |
| 14 | Ellagic acid galloyl hexoside | n.d. | 0.1415 | n.d. | 1.5511 |
| 15 | Rutin * | 0.3328 | 0.2804 | 3.0759 | 2.9646 |
| 16 | Quercetin 3-O-glucoside * | 0.0823 | 0.2332 | 0.9527 | 2.8943 |
| 17 | Quercetin 3-O-rhamnoside * | n.d. | 0.0971 | n.d. | 1.1118 |
| 18 | Kaempferol hexoside | 0.0176 | 0.0164 | 0.2496 | 0.2359 |
| 19 | Kaempferol 3-O-glucoside * | 0.0085 | n.d. | 0.1429 | n.d. |
| 20 | Myricetin * | 0.0366 | n.d. | 0.1950 | 0.6625 |
| 21 | Luteolin hexoside-deoxyhexoside | 0.0267 | n.d. | 0.1111 | n.d. |
| 22 | Methyl-quercetin | 0.3051 | n.d. | 1.1705 | 0.4556 |
| 23 | Methyl kaempferol, isomer 1 | 0.1902 | 0.4046 | 1.1919 | 2.9671 |
| 24 | Methyl kaempferol, isomer 2 | 0.0434 | n.d. | 0.0681 | n.d. |
| 25 | Methyl kaempferol, isomer 3 | 0.0120 | n.d. | 0.0129 | n.d. |
| 26 | Di-methyl quercetin isomer 1 | 0.1482 | n.d. | 0.0835 | n.d. |
| 27 | Luteolin * | 0.0172 | n.d. | 0.0076 | n.d. |
| 28 | Di-methyl quercetin isomer 2 | 0.1883 | n.d. | 0.1021 | n.d. |
| 29 | Di-methyl quercetin isomer 3 | 0.0308 | n.d. | 0.0080 | n.d. |
| 30 | Methyl apigenin isomer 1 | 0.0395 | n.d. | 0.0123 | n.d. |
| 31 | Methyl apigenin isomer 2 | 0.1017 | n.d. | 0.0294 | n.d. |
| 32 | Di-methyl kaempferol | 0.0448 | n.d. | 0.0059 | n.d. |
| 33 | Di-methyl quercetin derivative | 0.0298 | n.d. | n.d. | n.d. |
| 34 | Methyl luteolin | 0.0217 | n.d. | n.d. | n.d. |
| 35 | Di-methyl apigenin | 0.0322 | n.d. | 0.0117 | n.d. |
| 36 | Di-methyl kaempferol | 0.0074 | n.d. | n.d. | n.d. |
| Total polyphenols | 3.7371 ± 0.0017 | 2.9846 ± 0.0017 | 19.555 ± 0.0034 | 20.039 ± 0.0001 | |
| Total flavonoids | 3.4685 ± 0.0016 | 1.4385 ± 0.0012 | 19.004 ± 0.0029 | 17.017 ± 0.0002 | |
| Total hydroxycinnamic acids and derivatives | 0.2686 ± 0.0004 | 0.0253 ± 0.0003 | 0.5508 ± 00005 | - | |
| Total tannins | - | 1.5208 ± 0.0004 | - | 3.0236 ± 0.0003 | |
| Crude Extracts | Polyphenolic Fractions | |||
|---|---|---|---|---|
| Tests | CLLE | CSLE | CLLE PF | CSLE PF |
| DPPH (mmol TE/mg DE) | 0.551 ± 0.044 | 0.639 ± 0.046 | 1.284 ± 0.327 | 2.593 ± 0.572 a |
| ABTS (mmol TE/mg DE) | 2.072 ± 0.532 | 3.267 ± 0.776 | 2.668 ± 0.433 | 2.668 ± 0.433 |
| FRAP (mmol Fe2+E/mg DE) | 2.370 ± 0.290 | 8.41 ± 0.26 | 9.840 ± 0.620 | 11.520 ± 1.150 bc |
| Extract | IC50 mg/mL |
|---|---|
| CLLE | 0.126 (0.109–0.146) |
| CSLE | 0.067 (0.054–0.084) a |
| CLLE PF | 0.103 (0.087–0.121) |
| CSLE PF | 0.042 (0.033–0.052) a |
| Trolox | 0.05 (0.044–0.056) |
| CLLE | CSLE | CLLE PF | CSLE PF | |||||
|---|---|---|---|---|---|---|---|---|
| Microbial Strains | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) |
| S. aureusATCC 6538 | 250 | 2000 | 125 | 2000 | 250 | 2000 | 125 | 2000 |
| S. aureusATCC 43300 | 250 | 2000 | 125 | 2000 | 250 | 2000 | 125 | 2000 |
| S. epidermidisATCC 35984 | 125 | 2000 | 62.5 | 2000 | 125 | 2000 | 62.5 | 2000 |
| P. acnesATCC 11827 | 125 | 1000 | 125 | 500 | 125 | 1000 | 125 | 500 |
| P. aeruginosaDSM 102273 | 1000 | >2000 | 500 | >2000 | 1000 | >2000 | 500 | >2000 |
| E. coliATCC 10536 | 1000 | >2000 | 1000 | >2000 | 1000 | >2000 | 1000 | >2000 |
| K. pneumoniaeDSM 26371 | 2000 | >2000 | 1000 | >2000 | 2000 | >2000 | 1000 | >2000 |
| C. albicansATCC 10231 | 2000 | >2000 | 500 | >2000 | 2000 | >2000 | 500 | >2000 |
| LC50 (C.L. 95) at 48 h in NIH/3T3 cells (µg/mL) | 171 (140–210) | >250 | 78 (65–93) | 98 (90–108) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouabidi, M.; Salamone, F.L.; Gadhi, C.; Bouamama, H.; Speciale, A.; Ginestra, G.; Pulvirenti, L.; Siracusa, L.; Nostro, A.; Cristani, M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules 2023, 28, 2797. https://doi.org/10.3390/molecules28062797
Bouabidi M, Salamone FL, Gadhi C, Bouamama H, Speciale A, Ginestra G, Pulvirenti L, Siracusa L, Nostro A, Cristani M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules. 2023; 28(6):2797. https://doi.org/10.3390/molecules28062797
Chicago/Turabian StyleBouabidi, Maryem, Federica Lina Salamone, Chemseddoha Gadhi, Hafida Bouamama, Antonio Speciale, Giovanna Ginestra, Luana Pulvirenti, Laura Siracusa, Antonia Nostro, and Mariateresa Cristani. 2023. "Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities" Molecules 28, no. 6: 2797. https://doi.org/10.3390/molecules28062797
APA StyleBouabidi, M., Salamone, F. L., Gadhi, C., Bouamama, H., Speciale, A., Ginestra, G., Pulvirenti, L., Siracusa, L., Nostro, A., & Cristani, M. (2023). Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules, 28(6), 2797. https://doi.org/10.3390/molecules28062797