Insights into the Fluxional Processes of Monomethylcyclohexenyl Manganese Tricarbonyl
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Bonding
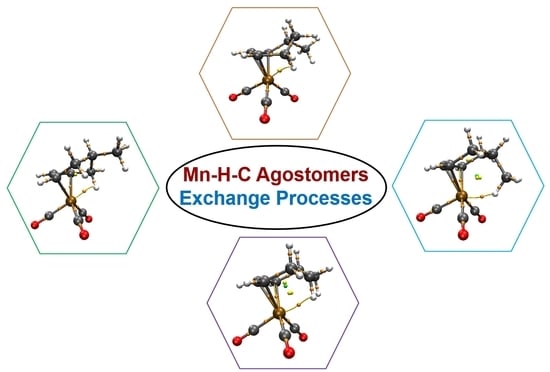
2.2. Exchange Processes
2.2.1. Isomerization of η3 Agostomers
2.2.2. Hydride Species Conversions
2.3. Interpretations of the Fluxionality
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brookhart, M.; Lukacs, A. Preparation of alkyl-substituted derivatives of cyclohexenylmanganese Tricarbonyl via Reduction of Methylated Arene Complexes. Organometallics 1983, 2, 649–658. [Google Scholar] [CrossRef]
- Brookhart, M.; Lamanna, W.; Humphrey, M.B. Structural Characterization and Fluxional Behavior of Cyclohexenylmanganese Tricarbonyl. Intramolecular C-H Bond Activation via a Two-Electron, Three-Center Mn...H...C Interaction. J. Am. Chem. Soc. 1982, 104, 2117–2126. [Google Scholar] [CrossRef]
- Schultz, A.J.; Teller, R.G.; Beno, M.A.; Williams, J.M.; Brookhart, M.; Lamanna, W.; Humphrey, M.B. Argonne Intense Pulsed Neutron Source Used to Solve the Molecular Structure of a Novel Organometallic Complex. Science 1983, 220, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.; Lamanna, W.; Pinhas, A.R. Synthesis and Reactivity of Cyclohexenylmanganese Tricarbonyl, a Complex Containing a Two-Electron, Three-Center Mn...H...C Interaction. Organometallics 1983, 2, 638–649. [Google Scholar] [CrossRef]
- Liang, G.; Webster, C.E. The Missing Agostomer in the Fluxionality of Cyclohexenyl Manganese Tricarbonyl. J. Organomet. Chem. 2018, 864, 128–135. [Google Scholar] [CrossRef]
- Himmelbauer, D.; Stöger, B.; Veiros, L.F.; Kirchner, K. Reversible Ligand Protonation of a Mn(I) PCP Pincer Complex To Afford a Complex with an η2-Caryl–H Agostic Bond. Organometallics 2018, 37, 3475–3479. [Google Scholar] [CrossRef]
- Saha, K.; Ramalakshmi, R.; Gomosta, S.; Pathak, K.; Dorcet, V.; Roisnel, T.; Halet, J.-F.; Ghosh, S. Design, Synthesis, and Chemistry of Bis(σ)borate and Agostic Complexes of Group 7 Metals. Chem. Eur. J. 2017, 23, 9812–9820. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, I.; Montag, M. Revisiting C–C and C–H Bond Activation in Rhodium Pincer Complexes: Thermodynamics and Kinetics Involving a Common Agostic Intermediate. Organometallics 2022, 41, 2379–2393. [Google Scholar] [CrossRef]
- Brookhart, M.; Green, M.L.; Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914. [Google Scholar] [CrossRef] [Green Version]
- Saßmannshausen, J. Agostic or not? Detailed Density Functional Theory studies of the compounds [LRh(CO)Cl], [LRh(COD)Cl] and [LRhCl] (L = cyclic (alkyl)(amino)carbene, COD = cyclooctadiene). Dalton Trans. 2011, 40, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, W.; Mo, Y. A theoretical perspective of the agostic effect in early transition metal compounds. Coord. Chem. Rev. 2020, 419, 213401. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Lu, T.; Chen, F. Bond Order Analysis Based on the Laplacian of Electron Density in Fuzzy Overlap Space. J. Phys. Chem. A 2013, 117, 3100–3108. [Google Scholar] [CrossRef]
- Solans-Monfort, X.; Eisenstein, O. DFT calculations of NMR JC–H coupling constants: An additional tool to characterize the α-agostic interaction in high oxidation state M-alkylidene complexes (M=Re, Mo and Ta). Polyhedron 2006, 25, 339–348. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in: Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Dunlap, B.I. Fitting the Coulomb potential variationally in Xα molecular calculations. J. Chem. Phys. 1983, 78, 3140–3142. [Google Scholar] [CrossRef]
- Dunlap, B.I. Robust and variational fitting: Removing the four-center integrals from center stage in quantum chemistry. J. Mol. Struct. THEOCHEM 2000, 529, 37–40. [Google Scholar] [CrossRef]
- Couty, M.; Hall, M.B. Basis sets for transition metals: Optimized outer p functions. J. Comput. Chem. 1996, 17, 1359–1370. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Check, C.E.; Faust, T.O.; Bailey, J.M.; Wright, B.J.; Gilbert, T.M.; Sunderlin, L.S. Addition of polarization and diffuse functions to the LANL2DZ basis set for p-block elements. J. Phys. Chem. A 2001, 105, 8111–8116. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular-Orbital Studies of Organic-Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. Influence of Polarization Functions on Molecular-Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian, Inc.: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, G. Understanding the Sigmatropic Shifts of Cyclopenta-2,4-dien-1-yltrimethylsilane in its Diels-Alder Addition. Org. Biomol. Chem. 2021, 19, 1732–1737. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Multiwfn, Version 3.8. 2021. Available online: https://sobereva.com/multiwfn/ (accessed on 15 December 2022).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Gr. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- VMD Version 1.9.3. 2016. Available online: https://www.ks.uiuc.edu/Research/vmd/ (accessed on 15 December 2022).
- Frey, N.C.; Dornshuld, E.V.; Webster, C.E. Benchmarking the Fluxional Processes of Organometallic Piano-Stool Complexes. Molecules 2021, 26, 2310. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. 1. Gauge-invariant LCAO method for N.M.R. chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A Comparison of Models for Calculating Nuclear Magnetic Resonance Shielding Tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised basis sets for the LANL effective core potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular-Orbital Methods 25. Supplementary Functions for Gaussian-Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]









| Structures | Mn-H (Å) | Mn-H-C (°) | C-H (Å) | H (ppm) | JCH (Hz) | |||
|---|---|---|---|---|---|---|---|---|
| endo | exo | endo | exo | endo | exo | |||
| 1 | 1.849 (1.860) | 97.6 (99.9) | 1.180 | 1.104 | −9.8 | 1.7 | 68.4 | 123.3 |
| 2 | 1.844 | 97.9 | 1.179 | 1.103 | −9.9 | 1.8 | 68.8 | 124.6 |
| 3 | 2.051 | 104.6 | 1.136 | 1.107 | −5.2 | 0.4 | 83.1 | 117.6 |
| 4 | 1.958 | 130.0 | 1.131 | 1.105 | −8.7 | 1.5 | 82.3 | 113.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, G.; Zhang, M. Insights into the Fluxional Processes of Monomethylcyclohexenyl Manganese Tricarbonyl. Molecules 2023, 28, 3232. https://doi.org/10.3390/molecules28073232
Liang G, Zhang M. Insights into the Fluxional Processes of Monomethylcyclohexenyl Manganese Tricarbonyl. Molecules. 2023; 28(7):3232. https://doi.org/10.3390/molecules28073232
Chicago/Turabian StyleLiang, Guangchao, and Min Zhang. 2023. "Insights into the Fluxional Processes of Monomethylcyclohexenyl Manganese Tricarbonyl" Molecules 28, no. 7: 3232. https://doi.org/10.3390/molecules28073232
APA StyleLiang, G., & Zhang, M. (2023). Insights into the Fluxional Processes of Monomethylcyclohexenyl Manganese Tricarbonyl. Molecules, 28(7), 3232. https://doi.org/10.3390/molecules28073232