Chemometrics of the Composition and Antioxidant Capacity of Hyptis crenata Essential Oils from Brazil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Chemical Composition of the Essential Oil
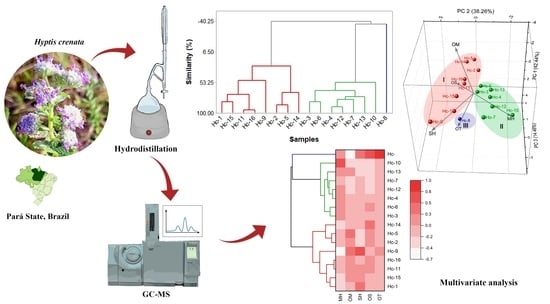
2.2. Multivariate Analyses of Hyptis crenata Specimens
2.3. Antioxidant Activity
3. Materials and Methods
3.1. Plant Material
3.2. Analysis of Essential Oil Composition
3.3. DPPH Radical Scavenging Assay
3.4. β-Carotene/linoleic Acid Assay
3.5. Multivariate Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Sample Code | Occurrence | Plant Part/ Extraction Type | Primary Components (>5%) | Oil Yield (%) | Ref. |
|---|---|---|---|---|---|
| Hc-7 | Mato Grosso do Sul, Brazil | Fresh material (HD) | camphor (17.3%), α-pinene (15.5%), (E)-caryophyllene (10.7%), β-pinene (10.5%), 1,8-cineol (8.7%), limonene (6.3%) | - | [18] |
| Hc-8 | Porto Nacional, Tocantins, Brazil | Dried aerial parts (HD) | terpinolene (37.8%), (E)-caryophyllene (9.9%), limonene (6.4%), α-pinene (6.1%) | 0.2 | [5] |
| Hc-9 | São Sebastião da Boa Vista, Pará, Brazil | Dried aerial parts (HD) | 1,8-cineole (23.9%), borneol (21.8%), (E)-caryophyllene (18.8%) | 0.9 | [5] |
| Hc-10 | Melgaço, Pará, Brazil | Dried aerial parts (HD) | α-pinene (51.1%), 1,8-cineole (16.5%) limonene (15.0), β-pinene (10.3%) | 0.9 | [5] |
| Hc-11 | Melgaço, Pará, Brazil | Dried aerial parts (HD) | 1,8-cineole (36.7%), α-pinene (14.5%), β-pinene (7.9%), α-terpineol (5.2%) | 0.6 | [5] |
| Hc-12 | Salvaterra, Pará, Brazil | Fresh aerial parts (HD) | α-pinene (22.0%), 1,8-cineole (17.6%), β-pinene (17.0%), limonene (5.4%) | 1.4 | [17] |
| Hc-13 | Salvaterra, Pará, Brazil | Dried aerial parts (HD) | 1,8-cineole (23.2%), α -pinene (19.5%), β-pinene (13.8%), camphor (11.6%), borneol (5.3%) | 0.9 | [17] |
| Hc-14 | Cuiabá, Mato Grosso, Brazil | Fresh aerial parts (HD) | borneol (17.8%), 1,8-cineole (15.6%), p-cymene (7.9%), γ-terpinene (5.3%) | 0.6 | [12] |
| Hc-15 | São Raimundo das Mangabeiras, Maranhão, Brazil | Fresh aerial parts (SD) | camphor (32.8%)1,8-cineole (18.0%), α-pinene (13.4%), (E)-caryophyllene (13.0%), p-cymene (5.4%) | - | [13] |
| Hc-16 | São Raimundo das Mangabeiras, Maranhão, Brazil | Fresh aerial parts (SD) | camphor (33.7), 1.8-cineole (19.8%), α-pinene (15.2%), and (E)-caryophyllene (8.0%), p-cymene (6.9), | - | [19] |
Appendix B
| Samples | MH | MO | SH | OS | OT | Reference |
|---|---|---|---|---|---|---|
| Hc-1 | 29.5 | 38.7 | 23.8 | 3.2 | 0.0 | * |
| Hc-2 | 41.8 | 49.8 | 3.3 | 3.9 | 0.1 | * |
| Hc-3 | 45.3 | 40.1 | 9.7 | 2.8 | 0.1 | * |
| Hc-4 | 56.9 | 33.7 | 5.4 | 1.7 | 0.1 | * |
| Hc-5 | 34.3 | 59.0 | 2.0 | 4.3 | 0.0 | * |
| Hc-6 | 51.8 | 31.2 | 8.5 | 5.0 | 0.0 | * |
| Hc-7 | 44.8 | 26.6 | 10.7 | 0.0 | 0.0 | [18] |
| Hc-8 | 58.7 | 1.6 | 20.0 | 9.8 | 5.3 | [5] |
| Hc-9 | 10.2 | 55.2 | 29.4 | 1.5 | 1.5 | [5] |
| Hc-10 | 80.4 | 18.1 | 1.4 | 0.0 | 0.0 | [5] |
| Hc-11 | 32.4 | 49.9 | 13.2 | 3.8 | 0.1 | [5] |
| Hc-12 | 57.8 | 25.4 | 6.6 | 1.3 | 0.0 | [17] |
| Hc-13 | 48.3 | 42.9 | 1.2 | 0.1 | 0.0 | [17] |
| Hc-14 | 22.6 | 40.9 | 5.5 | 6.8 | 0.0 | [12] |
| Hc-15 | 26.8 | 52.0 | 18.7 | 2.5 | 0.0 | [13] |
| Hc-16 | 31.0 | 53.4 | 11.9 | 3.7 | 0.0 | [19] |
Appendix C
| Sample | DPPH Assay | β-Carotene Assay | |
|---|---|---|---|
| Inhibition (%) * | TEAC (mg.TE/g) * | Inhibition (%) * | |
| Hc-1 | 19.2 ± 1.6 a | 214.8 ± 17.6 a | 16.4 ± 7.0 a |
| Hc-2 | 30.9 ± 0.3 b | 345.9 ± 3.0 b | 40.0 ± 2.4 b |
| Hc-3 | 23.5 ± 0.5 c | 262.7 ± 5.3 c | 29.4 ± 2.7 b,c,d |
| Hc-4 | 23.5 ± 0.7 c | 363.3 ± 7.4 c | 18.0 ± 1.6 a |
| Hc-5 | 49.3 ± 0.1 d | 551.9 ± 1.5 d | 26.7 ± 2.7 a,d |
| Hc-6 | 42.4 ± 0.9 e | 475.1 ± 10.3 e | 39.0 ± 1.9 b,c |
| Trolox | - | - | 81.8 ± 6.1 e |
References
- Zhao, F.; Chen, Y.-P.; Salmaki, Y.; Drew, B.T.; Wilson, T.C.; Scheen, A.-C.; Celep, F.; Bräuchler, C.; Bendiksby, M.; Wang, Q.; et al. An Updated Tribal Classification of Lamiaceae Based on Plastome Phylogenomics. BMC Biol. 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Bridi, H.; de Carvalho Meirelles, G.; Lino von Poser, G. Subtribe Hyptidinae (Lamiaceae): A Promising Source of Bioactive Metabolites. J. Ethnopharmacol. 2021, 264, 113225. [Google Scholar] [CrossRef] [PubMed]
- Sedano-Partida, M.D.; dos Santos, K.P.; Sala-Carvalho, W.R.; Silva-Luz, C.L.; Furlan, C.M. A Review of the Phytochemical Profiling and Biological Activities of Hyptis Jacq.: A Brazilian Native Genus of Lamiaceae. Braz. J. Bot. 2020, 43, 213–228. [Google Scholar] [CrossRef]
- WFO Hyptis Crenata Pohl Ex Benth. Available online: http://www.worldfloraonline.org/taxon/wfo-0000216589 (accessed on 10 March 2023).
- Maria das Graças, B.; Andrade, E.H.A.; da Silva, M.H.L.; Maia, J.G.S.; Luz, A.I.R.; da Silva, J.D. Chemical Variation in the Essential Oils of Hyptis Crenata Pohl Ex Benth. Flavour Fragr. J. 2002, 17, 5–8. [Google Scholar] [CrossRef]
- Harley, R.M.; Antar, G.M. Hyptis in Flora e Funga Do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB17875%3E (accessed on 1 April 2023).
- Di Stasi, L.C.; Oliveira, G.P.; Carvalhaes, M.A.; Queiroz-Junior, M.; Tien, O.S.; Kakinami, S.H.; Reis, M.S. Medicinal Plants Popularly Used in the Brazilian Tropical Atlantic Forest. Fitoterapia 2002, 73, 69–91. [Google Scholar] [CrossRef]
- Jesus, N.Z.T.D.; Lima, J.C.D.S.; Silva, R.M.D.; Espinosa, M.M.; Martins, D.T.D.O. Levantamento Etnobotânico de Plantas Popularmente Utilizadas Como Antiúlceras e Antiinflamatórias Pela Comunidade de Pirizal, Nossa Senhora Do Livramento-MT, Brasil. Rev. Bras. Farmacogn. 2009, 19, 130–139. [Google Scholar] [CrossRef] [Green Version]
- de Groot, A.C.; Schmidt, E. Tea Tree Oil: Contact Allergy and Chemical Composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Chizzola, R.; Michitsch, H.; Franz, C. Antioxidative Properties of Thymus Vulgaris Leaves: Comparison of Different Extracts and Essential Oil Chemotypes. J. Agric. Food Chem. 2008, 56, 6897–6904. [Google Scholar] [CrossRef]
- Das, S.; Sandeep, I.S.; Mohapatra, P.; Kar, B.; Sahoo, R.K.; Subudhi, E.; Nayak, S.; Mohanty, S. A Comparative Study of Essential Oil Profile, Antibacterial and Antioxidant Activities of Thirty Piper Betle Landraces towards Selection of Industrially Important Chemotypes. Ind. Crops Prod. 2022, 187, 115289. [Google Scholar] [CrossRef]
- Violante, I.M.P.; Garcez, W.S.; da Silva Barbosa, C.; Garcez, F.R. Chemical Composition and Biological Activities of Essential Oil from Hyptis Crenata Growing in the Brazilian Cerrado. Nat. Prod. Commun. 2012, 7, 1387–1389. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.R.L.; Vieira, C.F.X.; Dos Santos, E.C.; Lima, G.C.; Aragão, K.K.V.; Vasconcelos, R.P.; da Costa Araújo, P.C.; de Abreu Gomes Vasconcelos, Y.; Cunha de Oliveira, A.; de Oliveira, H.D.; et al. Gastroprotective Effects of the Essential Oil of Hyptis Crenata Pohl Ex Benth. on Gastric Ulcer Models. J. Ethnopharmacol. 2013, 149, 694–700. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.N.N.; Guimarães, B.A.; de Castro, A.L.S.; Ribeiro, K.B.; Miller, D.C.; da Silva, P.I.C.; Freitas, J.J.S.; de Lima, A.B.; Setzer, W.N.; da Silva, J.K.R.; et al. Chemical Composition and Antinociceptive and Anti-Inflammatory Activity of the Essential Oil of Hyptis Crenata Pohl Ex Benth. from the Brazilian Amazon. J. Ethnopharmacol. 2023, 300, 115720. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Rebelo, M.M.; da Silva, J.K.R.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant Capacity and Biological Activity of Essential Oil and Methanol Extract of Hyptis Crenata Pohl Ex Benth. Rev. Bras. Farmacogn. 2009, 19, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Scramin, S.; Saito, M.L.; Pott, A.; Marques, M.O.M. Volatile Constituents of Hyptis Crenata Pohl (Labiatae) Native in Brazilian Pantanal. J. Essent. Oil Res. 2000, 12, 99–101. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Alves-Soares, R.; Oliveira, H.D.; Gomes-Vasconcelos, Y.A.; Souza, P.J.C.; Santos-Nascimento, T.; Oliveira, K.A.; Diniz, L.R.L.; Guimarães-Pereira, J.; Leal-Cardoso, J.H. The Essential Oil of Hyptis Crenata Pohl Ex Benth. Presents an Antiedematogenic Effect in Mice. Braz. J. Med. Biol. Res. 2021, 54. [Google Scholar] [CrossRef]
- Lakušić, D.V.; Ristić, M.S.; Slavkovska, V.N.; Šinžar-Sekulić, J.B.; Lakušić, B.S. Environment-Related Variations of the Composition of the Essential Oils of Rosemary (Rosmarinus Officinalis L.) in the Balkan Penninsula. Chem. Biodivers. 2012, 9, 1286–1302. [Google Scholar] [CrossRef]
- Aissi, O.; Boussaid, M.; Messaoud, C. Essential Oil Composition in Natural Populations of Pistacia Lentiscus L. from Tunisia: Effect of Ecological Factors and Incidence on Antioxidant and Antiacetylcholinesterase Activities. Ind. Crops Prod. 2016, 91, 56–65. [Google Scholar] [CrossRef]
- Jaouadi, R.; Boussaid, M.; Zaouali, Y. Variation in Essential Oil Composition within and among Tunisian Thymus Algeriensis Boiss et Reut. (Lamiaceae) Populations: Effect of Ecological Factors and Incidence on Antiacetylcholinesterase and Antioxidant Activities. Biochem. Syst. Ecol. 2023, 106, 104543. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Mirzakhani, M.; Pirbalouti, A.G. Essential Oil Variation among 21 Wild Myrtle (Myrtus Communis L.) Populations Collected from Different Geographical Regions in Iran. Ind. Crops Prod. 2013, 51, 328–333. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Haldar, T.; Sahoo, A.; Kar, B.; Patnaik, J.; Ghosh, B.; Chandra Panda, P.; Mahapatra, N.; Nayak, S. Population Genetic Structure and Diversity Analysis in Hedychium Coronarium Populations Using Morphological, Phytochemical and Molecular Markers. Ind. Crops Prod. 2019, 132, 118–133. [Google Scholar] [CrossRef]
- Rawat, S.; Bhatt, I.D.; Rawal, R.S. Variation in Essential Oil Composition in Rhizomes of Natural Populations of Hedychium Spicatum in Different Environmental Condition and Habitats. J. Essent. Oil Res. 2020, 32, 348–360. [Google Scholar] [CrossRef]
- Holm, Y.; Laakso, I.; Hiltunen, R.; Galambosi, B. Variation in the Essential Oil Composition of Artemisia Annua L. of Different Origin Cultivated in Finland. Flavour Fragr. J. 1997, 12, 241–246. [Google Scholar] [CrossRef]
- Sugimoto, N.; Kiuchi, F.; Mikage, M.; Mori, M.; Mizukami, H.; Tsuda, Y. Pharmacognostical Investigation of Acori Rhizomes. Part III. DNA Profiling of Acorus Calamus Chemotypes Differing in Essential Oil Composition. Biol. Pharm. Bull. 1999, 22, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, H.J.; Bang, J.-H.; Chung, J.-W.; Hyun, T.K. Variation in Essential Oil Composition and Antimicrobial Activity among Different Genotypes of Perilla Frutescens Var. Crispa. J. Appl. Biol. Chem. 2021, 64, 127–131. [Google Scholar] [CrossRef]
- Moghaddam, M.; Miran, S.N.K.; Pirbalouti, A.G.; Mehdizadeh, L.; Ghaderi, Y. Variation in Essential Oil Composition and Antioxidant Activity of Cumin (Cuminum Cyminum L.) Fruits during Stages of Maturity. Ind. Crops Prod. 2015, 70, 163–169. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M. Variation in Essential Oil Composition, Bioactive Compounds, Anatomical and Antioxidant Activity of Achillea Aucheri, an Endemic Species of Iran, at Different Phenological Stages. Chem. Biodivers. 2018, 15, e1800319. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical Scavenging and Antioxidant Activities of Essential Oil Components—An Experimental and Computational Investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-Scavenging Activities of Citrus Essential Oils and Their Components: Detection Using 1,1-Diphenyl-2-Picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, Antioxidant Capacity and Cytotoxic Activity of Eugenia Uniflora L. Chemotype-Oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Mendonça, J.d.S.; Guimarães, R.d.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.d.C.; Hiane, P.A.; de Pádua Melo, E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Cardoso, R.V.; Santos, S.D.J.L.; de Araújo, A.L.; de Araújo, J.A.; Gandra, E.A. Efeito Da Extração Assistida Por Ultrassom Nos Teores de Compostos Fenólicos e Atividade Antioxidante de Extratos de Folha e Flor de Salva-Do-Marajó (Hyptis Crenata Pohl Ex Benth). Braz. J. Dev. 2020, 6, 61533–61542. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids From Brazil. Nat. Prod. Commun. 2021, 16, 1934578X2199615. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia Uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| RIC | RIL | Constituents | Hc-1 | Hc-2 | Hc-3 | Hc-4 | Hc-5 | Hc-6 |
|---|---|---|---|---|---|---|---|---|
| Samples (%) * | ||||||||
| 923 | 921 a | tricyclene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| 926 | 924 a | α-thujene | 0.0 | 0.4 | 0.1 | 0.1 | 0.3 | 0.2 |
| 934 | 932 a | α-pinene | 13.6 | 13.0 | 21.8 | 23.5 | 10.5 | 15.7 |
| 947 | 945 a | α-fenchene | 0.3 | 0.2 | 0.7 | 0.8 | 0.7 | |
| 949 | 946 a | camphene | 1.7 | 2.9 | 3.3 | 3.8 | 3.1 | 3.1 |
| 954 | 953 a | thuja-2,4(10)-diene | tr | 0.1 | tr | tr | ||
| 973 | 969 a | sabinene | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | |
| 978 | 974 a | β-pinene | 7.6 | 9.1 | 9.8 | 10.5 | 7.5 | 13.4 |
| 982 | 973 a | trans-p-menthane | 0.1 | |||||
| 991 | 988 a | myrcene | 0.9 | 1.6 | 2.4 | 2.2 | 1.3 | 2.3 |
| 1006 | 1002 a | α-phellandrene | 0.3 | 0.4 | 0.8 | 0.7 | 0.3 | 0.8 |
| 1011 | 1008 a | δ-3-carene | 0.2 | 0.7 | ||||
| 1017 | 1014 a | α-terpinene | 0.6 | 1.0 | 1.1 | 1.0 | 0.8 | 1.3 |
| 1024 | 1089 a | p-cymene | 0.8 | 2.9 | 1.7 | 2.0 | 2.2 | 2.0 |
| 1030 | 1024 a | limonene | 2.0 | 5.1 | 9.7 | 4.5 | 8.5 | |
| 1033 | 1026 a | 1,8-cineole | 31.0 | 19.2 | 31.5 | 23.5 | 18.4 | 17.4 |
| 1058 | 1054 a | γ-terpinene | 1.0 | 3.1 | 1.4 | 1.2 | 2.4 | 2.1 |
| 1066 | 1065 a | cis-sabinene hydrate (IPP vs. OH) | 0.2 | 0.1 | 0.2 | 0.1 | ||
| 1099 | 1098 a | trans-sabinene hydrate | 0.3 | |||||
| 1071 | 1067 a | cis-linalool oxide (furanoid) | 0.1 | |||||
| 1089 | 1086 a | terpinolene | 0.5 | 1.5 | 1.4 | 1.0 | 1.2 | 1.4 |
| 1100 | 1095 a | linalool | 0.1 | 0.5 | 0.2 | |||
| 1114 | 1114 a | endo-fenchol | 0.2 | 0.2 | 0.3 | 0.3 | 0.1 | 0.4 |
| 1121 | 1119 a | trans-pinene hydrate | 0.1 | 0.2 | 0.1 | |||
| 1121 | 1118 a | cis-p-menth-2-en-1-ol | 0.2 | 0.2 | ||||
| 1139 | 1136 a | trans-p-menth-2-en-1-ol | tr | 0.1 | 0.1 | |||
| 1144 | 1141 a | camphor | 1.9 | 17.6 | 2.4 | 3.8 | 19.3 | 4.5 |
| 1148 | 1145 a | camphene hydrate | 0.7 | 0.5 | 0.7 | 0.5 | 0.3 | 0.7 |
| 1157 | 1155 a | isoborneol | 1.4 | 0.1 | tr | 0.1 | ||
| 1162 | 1160 a | pinocarvone | tr | 0.1 | 0.1 | 0.2 | 0.2 | |
| 1166 | 1165 a | borneol | 6.7 | 1.6 | 1.8 | 16.4 | 2.3 | |
| 1177 | 1174 a | terpinen-4-ol | 0.5 | 1.3 | 0.8 | 0.8 | 1.1 | 1.2 |
| 1185 | 1179 a | p-cymen-8-ol | tr | 0.1 | 0.1 | |||
| 1191 | 1186 a | α-terpineol | 2.6 | 2.3 | 1.8 | 2.5 | 1.8 | 3.9 |
| 1197 | 1194 a | myrtenol | 0.1 | 0.3 | 0.4 | 0.4 | 0.2 | 0.5 |
| 1295 | 1289 a | thymol | tr | 0.3 | 0.1 | 0.2 | ||
| 1295 | 1297 a | carvacrol ethyl ether | 0.1 | 0.1 | ||||
| 1302 | 1298 a | carvacrol | tr | 0.1 | 0.1 | |||
| 1352 | 1350 a | α-longipinene | 5.2 | 0.2 | 1.0 | 0.9 | 0.1 | 1.0 |
| 1357 | 1356 a | eugenol | 0.1 | 0.1 | 0.1 | tr | ||
| 1372 | 1373 a | α-ylangene | 0.1 | 0.1 | ||||
| 1374 | 1374 a | isoledene | tr | tr | 0.1 | |||
| 1377 | 1374 a | α-copaene | 0.1 | 0.1 | ||||
| 1400 | 1407 a | longifolene | 0.1 | |||||
| 1411 | 1409 a | α-gurjunene | tr | 0.1 | ||||
| 1421 | 1417 a | (E)-caryophyllene | 7.8 | 1.2 | 2.9 | 2.0 | 0.9 | 4.3 |
| 1429 | 1439 a | aromadendrene | 1.1 | 1.0 | 1.1 | 0.9 | 0.6 | 1.4 |
| 1429 | 1430 b | γ-maaliene | 0.1 | 0.1 | 0.1 | tr | 0.1 | |
| 1435 | 1436 b | α-maaliene | 0.1 | 0.1 | 0.1 | 0.1 | tr | |
| 1444 | 1445 a | myltayl-4(12)-ene | 0.2 | |||||
| 1444 | 1545 b | selina-5,11-diene | 0.1 | 0.1 | 0.2 | |||
| 1450 | 1449 a | α-himachalene | 1.0 | 0.6 | 0.1 | 0.2 | ||
| 1454 | 1452 a | α-humulene | 0.4 | 0.1 | 0.2 | 0.1 | 0.3 | |
| 1462 | 1464 a | 9-epi-(E)-caryophyllene | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | |
| 1473 | 1475 a | γ-gurjunene | 0.1 | 0.1 | ||||
| 1479 | 1481 a | γ-himachalene | 1.4 | 0.8 | 0.2 | 0.2 | ||
| 1482 | 1485 a | 11α-himachala-1,4-diene | 0.5 | 0.1 | ||||
| 1487 | 1489 a | β-selinene | tr | 0.1 | ||||
| 1496 | 1496 a | viridiflorene | 0.5 | 0.4 | 0.5 | 0.3 | 0.2 | |
| 1502 | 1500 a | β-himachalene | 3.7 | 0.1 | 0.8 | 0.5 | 0.6 | |
| 1508 | 1511 a | δ-amorphene | 0.1 | |||||
| 1514 | 1516 a | α-dehydro-ar-himachalene | 0.4 | |||||
| 1515 | 1513 a | γ-cadinene | 0.2 | 0.1 | 0.2 | |||
| 1524 | 1522 a | δ-cadinene | 0.2 | 0.2 | 0.1 | |||
| 1529 | 1530 a | γ-dehydro-ar-himachalene | 0.3 | tr | ||||
| 1536 | 1540 b | selina-4(15),7(11)-diene | tr | 0.1 | ||||
| 1539 | 1545 a | selina-3,7(11)-diene | 0.1 | |||||
| 1543 | 1544 a | α-calacorene | 0.4 | |||||
| 1560 | 1562 a | epi-longipinanol | 0.1 | |||||
| 1567 | 1566 a | maaliol | 0.1 | tr | 0.1 | |||
| 1570 | 1570 a | caryophyllenyl alcohol | 0.1 | |||||
| 1578 | 1577 a | spathulenol | 0.1 | 0.4 | 0.2 | 0.2 | 0.2 | 0.3 |
| 1584 | 1585 a | caryophyllene oxide | 0.6 | 1.2 | 0.8 | 0.7 | 2.4 | |
| 1584 | 1590 a | globulol | 0.3 | 0.8 | 0.9 | |||
| 1592 | 1592 a | viridiflorol | 0.1 | 1.0 | 0.7 | 0.1 | 2.1 | 0.6 |
| 1597 | 1599 a | longiborneol | 0.1 | |||||
| 1602 | 1600 a | rosifoliol | 0.1 | 0.1 | 0.1 | 0.1 | ||
| 1613 | 1615 a | β-himachalene oxide | 0.1 | 0.1 | 0.1 | |||
| 1616 | 1618 a | 1,10-di-epi-cubenol | 0.1 | 0.1 | ||||
| 1620 | 1622 a | 10-epi-γ-eudesmol | 0.5 | 0.7 | ||||
| 1620 | 1618 a | junenol | 0.1 | |||||
| 1637 | 1639 a | caryophylla-4(12),8(13)-dien-5β-ol | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.5 |
| 1645 | 1640 a | hinesol | 0.3 | 0.2 | ||||
| 1646 | 1652 a | himachalol | 0.6 | 0.5 | ||||
| 1653 | 1656 a | valerianol | 0.2 | 0.3 | ||||
| 1658 | 1668 a | 14-hydroxy-9-epi-(E)-caryophyllene | 1.1 | |||||
| 1662 | 1661 a | allohimachalol | 0.1 | 0.1 | ||||
| 1675 | 1675 a | cadalene | 0.1 | |||||
| Monoterpene hydrocarbons | 29.5 | 41.8 | 45.3 | 56.9 | 34.3 | 51.8 | ||
| Oxygenated monoterpenes | 38.7 | 49.8 | 40.1 | 33.7 | 59.0 | 31.2 | ||
| Sesquiterpene hydrocarbons | 23.8 | 3.3 | 9.7 | 5.4 | 2.0 | 8.5 | ||
| Oxygenated sesquiterpenes | 3.2 | 3.9 | 2.8 | 1.7 | 4.3 | 5.0 | ||
| Other compounds | tr | 0.1 | 0.1 | 0.1 | tr | tr | ||
| Total (%) | 95.1 | 98.9 | 97.9 | 97.8 | 99.7 | 96.5 | ||
| Oil yield (%, v/w) | 1.6 | 2.8 | 1.9 | 1.7 | 1.1 | 3.1 | ||
| Code | Collection Site | Voucher Number | Coordinates Latitude/Longitude |
|---|---|---|---|
| Hc-1 | Salvaterra, Marajó, Pará state, Brazil | MG243648 | 1°51′43.71″ S/48°37′23.33″ W |
| Hc-2 | Cachoeira do Arari, Marajó, Pará state, Brazil | MG238838 | 0°54′27.77″ S/48°40′30.45″ W |
| Hc-3 | Salvaterra, Marajó, Pará state, Brazil | MG246271 | 0°52′7.04″ S/48°37′38.06″ W |
| Hc-4 | Salvaterra, Marajó, Pará state, Brazil | MG246272 | 0°51′52.72″ S/48°37′9.69″ W |
| Hc-5 | Salvaterra, Marajó, Pará state, Brazil | MG238839 | 0°51′42.71″ S/48°37′23.87″ W |
| Hc-6 | Cachoeira do Arari, Marajó, Pará state, Brazil | MG238843 | 0°54′27.74″ S/48°40′3.51″ W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.N.N.d.; Costa, J.S.d.; Guimarães, B.A.; Freitas, J.J.S.; Setzer, W.N.; Silva, J.K.R.d.; Maia, J.G.S.; Figueiredo, P.L.B. Chemometrics of the Composition and Antioxidant Capacity of Hyptis crenata Essential Oils from Brazil. Molecules 2023, 28, 3371. https://doi.org/10.3390/molecules28083371
Lima MNNd, Costa JSd, Guimarães BA, Freitas JJS, Setzer WN, Silva JKRd, Maia JGS, Figueiredo PLB. Chemometrics of the Composition and Antioxidant Capacity of Hyptis crenata Essential Oils from Brazil. Molecules. 2023; 28(8):3371. https://doi.org/10.3390/molecules28083371
Chicago/Turabian StyleLima, Maria Nancy N. de, Jamile Silva da Costa, Bruna A. Guimarães, Jofre Jacob S. Freitas, William N. Setzer, Joyce Kelly R. da Silva, José Guilherme S. Maia, and Pablo Luis B. Figueiredo. 2023. "Chemometrics of the Composition and Antioxidant Capacity of Hyptis crenata Essential Oils from Brazil" Molecules 28, no. 8: 3371. https://doi.org/10.3390/molecules28083371
APA StyleLima, M. N. N. d., Costa, J. S. d., Guimarães, B. A., Freitas, J. J. S., Setzer, W. N., Silva, J. K. R. d., Maia, J. G. S., & Figueiredo, P. L. B. (2023). Chemometrics of the Composition and Antioxidant Capacity of Hyptis crenata Essential Oils from Brazil. Molecules, 28(8), 3371. https://doi.org/10.3390/molecules28083371