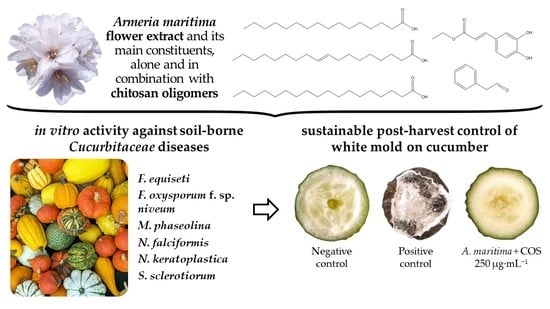
Armeria maritima (Mill.) Willd. Flower Hydromethanolic Extract for Cucurbitaceae Fungal Diseases Control
Abstract
:1. Introduction
2. Results
2.1. Vibrational Spectroscopy Characterization
2.2. GC–MS Characterization
2.3. Antifungal Activity of the Extract
2.3.1. In Vitro Antifungal Activity
2.3.2. Ex Situ Antifungal Activity
3. Discussion
3.1. On the Phytochemical Profile Obtained by GC–MS
3.2. On the Antifungal Activity and Mode of Action
3.3. Efficacy Comparisons
3.3.1. Comparison with Conventional Fungicides
3.3.2. Comparison with Other Extracts Tested In Vitro against the Phytopathogens under Study
3.3.3. Comparison with Other Extracts Tested Ex Situ for Cucumber Protection
3.4. Limitations of the Study and Further Research
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. Phytopathogen Isolates
4.3. Preparation of Armeria Extract, Chitosan Oligomers, and Conjugate Complexes
4.4. Physicochemical Characterization
4.5. In Vitro Antifungal Activity Assessment
4.6. Post-Harvest Protection Test in Cucumber
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Woodell, S.R.J.; Dale, A. Armeria Maritima (Mill.) Willd. (Statice armeria L.; S. maritima Mill.). J. Ecol. 1993, 81, 573. [Google Scholar] [CrossRef]
- Brewin, L.; Mehra, A.; Lynch, P.; Farago, M. Mechanisms of copper tolerance by Armeria maritima in Dolfrwynog Bog, North Wales–Initial studies. Environ. Geochem. Health 2003, 25, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gourguillon, L.; Cattuzzato, L.; Lavaud, C.; Lobstein, A. Biological properties and phytochemical analysis of the halophyte Armeria maritima Willd. (Plumbaginaceae). Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Lauranson, J.; Vekemans, X.; Lefebvre, C.; Jay, M. Flavonoid profiles variation in Armeria maritima (Mill.) Willd. Biochem. Syst. Ecol. 1995, 23, 319–329. [Google Scholar] [CrossRef]
- Stewart, G.R.; Lee, J.A. The role of proline accumulation in halophytes. Planta 1974, 120, 279–289. [Google Scholar] [CrossRef]
- Romero, M.; Blundem, G. Quaternary ammonium compounds found in stressed plants. Rev. Fitoter. 2002, 2, 334. [Google Scholar]
- Gourguillon, L.; Zumbiehl, C.; Antheaume, C.; Lobstein, A. First isolation and identification of polyphenols from the halophyte Armeria maritima. Planta Med. 2015, 81, PM_65. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of Scottish plants for antibacterial activity. J. Ethnopharmacol. 2002, 83, 73–77. [Google Scholar] [CrossRef]
- Gourguillon, L.; Rustenholz, C.; Lobstein, A.; Gondet, L. Callus induction and establishment of cell suspension cultures of the halophyte Armeria maritima (Mill.) Willd. Sci. Hortic. 2018, 233, 407–411. [Google Scholar] [CrossRef]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2020, 226, 1240–1255. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Paris, H.S. Melons, squashes, and gourds. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3817–3826. [Google Scholar] [CrossRef]
- Chehri, K.; Salleh, B.; Yli-Mattila, T.; Reddy, K.; Abbasi, S. Molecular characterization of pathogenic Fusarium species in cucurbit plants from Kermanshah province, Iran. Saudi J. Biol. Sci. 2011, 18, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.P.; Wechter, W.P.; Rutter, W.B.; Agudelo, P.A. Cucurbit rootstocks resistant to Fusarium oxysporum f. sp. niveum remain resistant when coinfected by Meloidogyne incognita in the field. Plant Dis. 2019, 103, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Martyn, R.D. Fusarium wilt of Watermelon, 2nd ed.; Keinath, A.P., Wintermantel, W.M., Zitter, T.A., Eds.; American Phytopathology Society: St. Paul, MN, USA, 2017. [Google Scholar]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, D.; Sharma, M.; Tarafdar, A.; Gupta, M. First report of Fusarium equiseti causing crown and root rot of cucumber in India. J. Plant Pathol. 2022, 104, 875. [Google Scholar] [CrossRef]
- de Sousa Linhares, C.M.; Ambrósio, M.M.Q.; Castro, G.; Torres, S.B.; Esteras, C.; de Sousa Nunes, G.H.; Picó, B. Effect of temperature on disease severity of charcoal rot of melons caused by Macrophomina phaseolina: Implications for selection of resistance sources. Eur. J. Plant Pathol. 2020, 158, 431–441. [Google Scholar] [CrossRef]
- Cohen, R.; Elkabetz, M.; Paris, H.S.; Freeman, S.; Gur, A. Charcoal rot (Macrophomina phaseolina) across melon diversity: Evaluating the interaction between the pathogen, plant age and environmental conditions as a step towards breeding for resistance. Eur. J. Plant Pathol. 2022, 163, 601–613. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, M.S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.M.; Hossain, M.Z.; Ahmed, B.; Rahim, S.; Rahman, M.S. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Lombard, L.; Crous, P. Back to the roots: A reappraisal of Neocosmospora. Pers. Mol. Phylogeny Evol. Fungi 2019, 43, 90–185. [Google Scholar] [CrossRef]
- Cabral, C.; Melo, M.; Fonseca, M.; Boiteux, L.; Reis, A. A root rot of chickpea caused by isolates of the Fusarium solani species complex in Brazil. Plant Dis. 2016, 100, 2171. [Google Scholar] [CrossRef]
- Sousa, E.; Melo, M.; Mota, J.; Sousa, E.; Beserra, J., Jr.; Matos, K. First report of Fusarium falciforme (FSSC 3 + 4) causing root rot in lima bean (Phaseolus lunatus L.) in Brazil. Plant Dis. 2017, 101, 1954. [Google Scholar] [CrossRef]
- Crespo, M.; Lawrence, D.P.; Nouri, M.T.; Doll, D.A.; Trouillas, F.P. Characterization of Fusarium and Neocosmospora species associated with crown rot and stem canker of pistachio rootstocks in California. Plant Dis. 2019, 103, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Ramírez, M.; López-Orona, C.; Velázquez-Alcaraz, T.d.J.; Díaz-Valdés, T.; Velarde-Félix, S.; Martínez-Campos, A.; Retes-Manjarrez, J. First report of onion basal rot caused by Fusarium falciforme in Mexico. Plant Dis. 2018, 102, 2646. [Google Scholar] [CrossRef]
- Rentería-Martínez, M.E.; Guerra-Camacho, M.Á.; Ochoa-Meza, A.; Moreno-Salazar, S.F.; Varela-Romero, A.; Gutiérrez-Millán, L.E.; Meza-Moller, A.d.C. Multilocus phylogenetic analysis of fungal complex associated with root rot watermelon in Sonora, Mexico. Rev. Mex. Fitopatol. 2018, 36, 233–255. [Google Scholar] [CrossRef]
- González, V.; Ruiz, J.; Picó, B.; García-Martínez, S.; Garcés-Claver, A.; Flores-León, A. First report of Neocosmospora falciformis causing wilt and root rot of muskmelon in Spain. Plant Dis. 2020, 104, 4. [Google Scholar] [CrossRef]
- González, V.; García-Martínez, S.; Flores-León, A.; Ruiz, J.; Picó, B.; Garcés-Claver, A. Neocosmospora keratoplastica, a relevant human fusarial pathogen is found to be associated with wilt and root rot of muskmelon and watermelon crops in Spain: Epidemiological and molecular evidences. Eur. J. Plant Pathol. 2020, 156, 1189–1196. [Google Scholar] [CrossRef]
- Kim, W.-G.; Cho, W.-D.; Jee, H.-J. Occurrence of Sclerotinia rot on cucurbitaceous vegetable crops in greenhouses. Kor. J. Mycol. 1999, 27, 198–205. [Google Scholar]
- Rahman, M.; Suzuki, K.; Islam, M.; Dey, T.; Harada, N.; Hossain, D. Molecular characterization, mycelial compatibility grouping, and aggressiveness of a newly emerging phytopathogen, Sclerotinia sclerotiorum, causing white mold disease in new host crops in Bangladesh. J. Plant Pathol. 2020, 102, 775–785. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Xiang, Y.; Du, L.; Huang, X.; Liu, Y. Comparative transcriptome analysis of Sclerotinia sclerotiorum revealed its response mechanisms to the biological control agent, Bacillus amyloliquefaciens. Sci. Rep. 2020, 10, 12576. [Google Scholar] [CrossRef]
- Lin, D.L.; Wang, S.M.; Wu, C.H.; Chen, B.G.; Liu, R.H. Chemical derivatization for the analysis of drugs by GC-MS—A conceptual review. J. Food Drug Anal. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of conjugate complexes of chitosan and Urtica dioica or Equisetum arvense extracts for the control of grapevine trunk pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Langa-Lomba, N.; Casanova-Gascón, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Characterization and antimicrobial activity of a halophyte from the Asturian coast (Spain): Limonium binervosum (G.E.Sm.) C.E.Salmon. Plants 2021, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Ravi, L.; Krishnan, K. Research article cytotoxic potential of N-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J. Cell Biol 2017, 12, 20–27. [Google Scholar] [CrossRef]
- Lalhruaizela, L.; Marak, B.N.; Gogoi, D.; Dowarah, J.; Sran, B.S.; Pachuau, Z.; Singh, V.P. Study of supramolecular self-assembly of pyridone and dihydropyridone co-crystal: Synthesis, crystal structure, Hirshfeld surface, DFT and molecular docking studies. J. Mol. Struct. 2021, 1235, 130214. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Murugan, M.; Kalaimathi, R.; Krishnaveni, K.; Basha, A.; Gilles, A.P.; Kandeepan, C.; Senthilkumar, N.; Mathialagan, B.; Ramya, S.; Jayakumararaj, R. ADMETox-informatics of plant derived octadecanoic acid (stearic acid) from ethyl acetate fraction of Moringa oleifera leaf extract as a natural lead for next generation drug design, development and therapeutics. J. Drug Deliv. Ther. 2022, 12, 129–141. [Google Scholar] [CrossRef]
- Ponnamma, S.; Manjunath, K. GC-MS analysis of phytocomponents in the methanolic extract of Justicia wynaadensis (Nees) T. Anders. Int. J. Pharma Bio Sci. 2012, 3, 570–576. [Google Scholar]
- Nalina, T.; Rahim, Z. The crude aqueous extract of Piper betle L. and its antibacterial effect towards Streptococcus mutans. Am. J. Biotechnol. Biochem. 2007, 3, 10–15. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Doyle, E. Trans fatty acids. J. Chem. Educ. 1997, 74, 1030. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-I.; Chang, K.-S.; Ahn, Y.-J. Repellent activity of constituents identified in Foeniculum vulgare fruit against Aedes aegypti (Diptera: Culicidae). J. Agric. Food. Chem. 2002, 50, 6993–6996. [Google Scholar] [CrossRef] [PubMed]
- Garba, S.; Garba, I. Anti-diarrhoeal properties of cis-9-octadecenoic acid isolated from Landolphia owariensis plant. Org. Med. Chem. IJ 2017, 3, 103. [Google Scholar]
- Zhang, Y.; Shi, S.; Wang, Y.; Huang, K. Target-guided isolation and purification of antioxidants from Selaginella sinensis by offline coupling of DPPH-HPLC and HSCCC experiments. J. Chromatogr. B 2011, 879, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.-W.; Jiang, J.-G. Functional analyses on antioxidant, anti-inflammatory, and antiproliferative effects of extracts and compounds from Ilex latifolia Thunb., a Chinese bitter tea. J. Agric. Food. Chem. 2014, 62, 8608–8615. [Google Scholar] [CrossRef]
- Qian, C.-Y.; Quan, W.-X.; Xiang, Z.-M.; Li, C.-C. Characterization of volatile compounds in four different rhododendron flowers by GC×GC-QTOFMS. Molecules 2019, 24, 3327. [Google Scholar] [CrossRef]
- Kala, S.; Ammani, K. GC-MS analysis of biologically active compounds in Canthium parviflorum Lam. leaf and callus extracts. Int. J. Chemtech Res. 2017, 10, 1039–1058. [Google Scholar]
- Tanapichatsakul, C.; Monggoot, S.; Gentekaki, E.; Pripdeevech, P. Antibacterial and antioxidant metabolites of Diaporthe spp. isolated from flowers of Melodorum fruticosum. Curr. Microbiol. 2017, 75, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, N.; Kumaran, N.S. Protective effect of Lawsonia inermis Linn. on chronic inflammation in rats. Int. J. Green Pharm. 2018, 12, 549–554. [Google Scholar]
- Kouzai, Y.; Noutoshi, Y.; Inoue, K.; Shimizu, M.; Onda, Y.; Mochida, K. Benzothiadiazole, a plant defense inducer, negatively regulates sheath blight resistance in Brachypodium distachyon. Sci. Rep. 2018, 8, 17358. [Google Scholar] [CrossRef] [PubMed]
- Aubert, S. Methyl-β-D-glucopyranoside in higher plants: Accumulation and intracellular localization in Geum montanum L. leaves and in model systems studied by 13C nuclear magnetic resonance. J. Exp. Bot. 2004, 55, 2179–2189. [Google Scholar] [CrossRef]
- Martin-Ramos, P.; Martin-Gil, J.; Gomez-Garcia, D.; Cuchi-Oterino, J.A. On the physicochemical characteristics and applications of an “undesirable” Pyrenean thorny cushion dwarf: Echinospartum horridum (Vahl) Roth. Plants 2020, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Ghavam, M.; Afzali, A.; Manca, M.L. Chemotype of Damask rose with oleic acid (9 octadecenoic acid) and its antimicrobial effectiveness. Sci. Rep. 2021, 11, 8027. [Google Scholar] [CrossRef] [PubMed]
- Hajji, A.; Bnejdi, F.; Saadoun, M.; Ben Salem, I.; Nehdi, I.; Sbihi, H.; Alharthi, F.A.; El Bok, S.; Boughalleb-M’Hamdi, N. High reserve in δ-tocopherol of Peganum harmala seeds oil and antifungal activity of oil against ten plant pathogenic fungi. Molecules 2020, 25, 4569. [Google Scholar] [CrossRef] [PubMed]
- Altieri, C.; Bevilacqua, A.; Cardillo, D.; Sinigaglia, M. Antifungal activity of fatty acids and their monoglycerides against Fusarium spp. in a laboratory medium. Int. J. Food Sci. Technol. 2009, 44, 242–245. [Google Scholar] [CrossRef]
- Abubacker, M.; Deepalakshmi, T. In vitro antifungal potential of bioactive compound methyl ester of hexadecanoic acid isolated from Annona muricata Linn (Annonaceae) leaves. Biosci. Biotechnol. Res. Asia 2013, 10, 879–884. [Google Scholar] [CrossRef]
- Avis, T.J.; Bélanger, R.R. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.l.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Hada, T.; Uryu, K.; Tsuzuki, T.; Eitsuka, T.; Miyazawa, T.; Murakami-Nakai, C.; Yoshida, H.; Mizushina, Y. Inhibitory effect of conjugated eicosapentaenoic acid on mammalian DNA polymerase and topoisomerase activities and human cancer cell proliferation. Biochem. Pharmacol. 2005, 70, 453–460. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The potential of fatty acids and their derivatives as antifungal agents: A review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, X.; Zhu, C.; Hu, F.; Hui, J. Inhibitory effect and mechanisms of sophorolipids against Staphylococcus aureus. J. Food Sci. 2012, 33, 33–36. [Google Scholar]
- Ginsburg, I.; van Heerden, P.; Koren, E. From amino acids polymers, antimicrobial peptides, and histones, to their possible role in the pathogenesis of septic shock: A historical perspective. J. Inflamm. Res. 2017, 10, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, J.; Zhang, P.; Xie, S.; Yuan, X.; Hou, X.; Yan, N.; Fang, Y.; Du, Y. In vitro and in vivo antifungal activity and preliminary mechanism of cembratrien-diols against Botrytis cinerea. Ind. Crops Prod. 2020, 154, 112745. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid conjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Correa-Guimarães, A.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical profile and activity against Fusarium species of Tamarix gallica bark aqueous ammonia extract. Agronomy 2023, 13, 496. [Google Scholar] [CrossRef]
- Naeini, A.; Ziglari, T.; Shokri, H.; Khosravi, A.R. Assessment of growth-inhibiting effect of some plant essential oils on different Fusarium isolates. J. Mycol. Med. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Rahman, M.; Bhuiyan, M.; Bhuiyan, M.; Khandaker, M. In vitro integrated management of blossom blight disease of tuberose (Polianthes tuberosa L.) caused by Fusarium equiseti. Pak. J. Phytopathol. 2012, 24, 48–52. [Google Scholar]
- Chacón, C.; Bojórquez-Quintal, E.; Caamal-Chan, G.; Ruíz-Valdiviezo, V.M.; Montes-Molina, J.A.; Garrido-Ramírez, E.R.; Rojas-Abarca, L.M.; Ruiz-Lau, N. In vitro antifungal activity and chemical composition of Piper auritum Kunth essential oil against Fusarium oxysporum and Fusarium equiseti. Agronomy 2021, 11, 1098. [Google Scholar] [CrossRef]
- Hossain, M.M.; Mazumder, K.; Hossen, S.M.; Tanmy, T.T.; Rashi, M.J. In vitro studies on antibacterial and antifungal activities of Emblica officinalis. Int. J. Pharm. Sci. Res. 2012, 3, 1124. [Google Scholar] [CrossRef]
- Anwar, M.N.; Rahman, M.S. Antimicrobial activity of crude extract obtained from the root of Plumbago zeylanica. Bangladesh J. Microbiol. 1970, 24, 73–75. [Google Scholar] [CrossRef]
- Satish, S.; Raghavendra, M.P.; Raveesha, K.A. Antifungal potentiality of some plant extracts against Fusarium sp. Arch. Phytopathol. Plant Prot. 2009, 42, 618–625. [Google Scholar] [CrossRef]
- Nelson, D.; Beattie, K.; McCollum, G.; Martin, T.; Sharma, S.; Rao, J.R. Performance of natural antagonists and commercial microbiocides towards in vitro suppression of flower bed soil-borne Fusarium oxysporum. Adv. Microbiol. 2014, 4, 151–159. [Google Scholar] [CrossRef]
- Cherkupally, R.; Kota, S.R.; Amballa, H.; Reddy, B.N. In vitro antifungal potential of plant extracts against Fusarium oxysporum, Rhizoctonia solani and Macrophomina phaseolina. Ann. Plant Sci. 2017, 6, 1676. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2016, 147, 229–238. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Rahman, A.; Ahmed, Y.; Kang, S.C. Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Cestrum nocturnum L. Pestic. Biochem. Physiol. 2010, 96, 86–92. [Google Scholar] [CrossRef]
- Chakma, M.; Bashar, M.A. In vitro control of Fusarium solani and F. oxysporum the causative agent of brinjal wilt. Dhaka Univ. J. Biol. Sci. 2014, 23, 53–60. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Hernández-Castillo, D.; Angulo-Sánchez, J.L.; Rodríguez-García, R.; Villarreal Quintanilla, J.A.; Lira-Saldivar, R.H. Antifungal activity in vitro of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani, and Fusarium oxysporum. Ind. Crops Prod. 2007, 25, 111–116. [Google Scholar] [CrossRef]
- El-Mohamedy, R.S.R.; Abdalla, A.M. Evaluation of antifungal activity of Moringa oleifera extracts as natural fungicide against some plant pathogenic fungi in-vitro. Int. J. Agric. Technol. 2014, 10, 963–982. [Google Scholar]
- Al-Araji, A.M.Y.; Hasan, A.M.; Fradi, A.J.; Majeed, S.M.A.; Majeed, W.W.A.; Naji, E.T. Study of alkaloids, phenols and terpenes of Mentha spicata as a fungicide against Rhizoctonia solani, Sclerotinia sclerotiorum and Fusarium oxysporum. Int. J. Adv. Biol. Res. 2017, 7, 345–354. [Google Scholar]
- Singh, U.P.; Pathak, K.K.; Khare, M.N.; Singh, R.B. Effect of leaf extract of garlic on Fusarium oxysporum f. sp. ciceri, Sclerotinia sclerotiorum and on gram seeds. Mycologia 2018, 71, 556–564. [Google Scholar] [CrossRef]
- Rashmi, S.; Rajkumar, H.G. Phytochemical analysis and in vitro evaluation of antifungal activity of five invasive plant species against Macrophomina Phaseolina (Tassi) Goid. Int. J. Plant Res. 2012, 1, 11–15. [Google Scholar] [CrossRef]
- Balamurugan, S. In vitro antifungal activity of Citrus aurantifolia Linn plant extracts against phytopathogenic fungi Macrophomina phaseolina. Int. Lett. Nat. Sci. 2014, 13, 70–74. [Google Scholar] [CrossRef]
- Dutta, P.; Sarma, N.; Saikia, S.; Gogoi, R.; Begum, T.; Lal, M. Pharmacological activity of Trachyspermum ammi L. seeds essential oil grown from Northeast India. J. Essent. Oil Bear. Plants 2022, 24, 1373–1388. [Google Scholar] [CrossRef]
- Begum, T.; Gogoi, R.; Sarma, N.; Pandey, S.K.; Lal, M. Direct sunlight and partial shading alter the quality, quantity, biochemical activities of Kaempferia parviflora Wall., ex Baker rhizome essential oil: A high industrially important species. Ind. Crops Prod. 2022, 180, 114765. [Google Scholar] [CrossRef]
- Pandey, S.K.; Gogoi, R.; Bhandari, S.; Sarma, N.; Begum, T.; Munda, S.; Lal, M. A comparative study on chemical composition, pharmacological potential and toxicity of Pogostemon cablin Linn., (Patchouli) flower and leaf essential oil. J. Essent. Oil Bear. Plants 2022, 25, 160–179. [Google Scholar] [CrossRef]
- Krumova, E.; Nikolova, M.; Miteva-Staleva, J.; Kostadinova, N.; Abrashev, R.; Dishliyska, V.; Berkov, S.; Mutafova, B.; Angelova, M. Bio-efficacy of the essential oil isolated from Origanum vulgare subsp. hirtum against fungal pathogens of potato. C. R. Acad. Bulg. Sci. 2021, 74, 1571–1578. [Google Scholar] [CrossRef]
- Ahmed, G.; Elsisi, A. Efficacy of compost and some essential oils alone or in combination in controlling cucumber white mould disease under protected house conditions J. Plant Prot. Pathol. 2020, 11, 291–297. [Google Scholar] [CrossRef]
- Elbatawy, Y.M.; Mohamed, F.G.; Eisa, N.A.; El-Habbak, M.H. Evaluation of some biological agents and plant extracts for controlling cucumber white rot caused by Sclerotinia sclerotiorum (Lib) de Bary. Ann. Agric. Sci. Moshtohor 2020, 58, 351–364. [Google Scholar] [CrossRef]
- Hussein, K.A. Antifungal activity and chemical composition of ginger essential oil against ginseng pathogenic fungi. Curr. Res. Environ. Appl. Mycol. 2018, 8, 194–203. [Google Scholar] [CrossRef]
- Onaran, A.; Yılar, M. Antifungal and herbicidal activity of Trachystemon orientalis (L.) G. Don against some plant pathogenic fungi and Cuscuta campestris Yunck. Iğdır Univ. J. Inst. Sci. Technol. 2018, 8, 37–43. [Google Scholar] [CrossRef]
- Goussous, S.J.; Mas’ad, I.S.; Abu El-Samen, F.M.; Tahhan, R.A. In vitro inhibitory effects of rosemary and sage extracts on mycelial growth and sclerotial formation and germination of Sclerotinia sclerotiorum. Arch. Phytopathol. Plant Prot. 2013, 46, 890–902. [Google Scholar] [CrossRef]
- Onaran, A.; Yanar, Y. In vivo and in vitro antifungal activities of five plant extracts against various plant pathogens. Egypt. J. Biol. Pest Control 2016, 26, 405–411. [Google Scholar]
- Onaran, A.; Bayar, Y.; Karakurt, T.; Tokatlı, K.; Bayram, M.; Yanar, Y. Antifungal activity of chitosan against soil-borne plant pathogens in cucumber and a molecular docking study. J. Taibah Univ. Sci. 2021, 15, 852–860. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Santiago-Aliste, A.; Torres-Sánchez, S.; Martín-Ramos, P. Lignin–chitosan nanocarriers for the delivery of bioactive natural products against wood-decay phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Martín-Gil, J.; Lorenzo-Vidal, B.; Palacio-Bielsa, A.; Martín-Ramos, P. Phytochemical screening and antibacterial activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 2023, 9, 201. [Google Scholar] [CrossRef]





| Flowers | Root | Stem | Assignment |
|---|---|---|---|
| 3290 | 3282 | 3355 | OH group in phenolic compounds |
| 2918 | 2921 | 2919 | O−H stretching |
| 2850 | 2851 | 2850 | –CH2 symmetric stretching (cutine and wax); CH2–(C6)– bending (cellulose) |
| 1732 | 1726 | C=O stretching of alkyl ester | |
| 1651 | C=O (amide I) | ||
| 1633 | 1620 | 1639 | skeletal vibration due to aromatic C=C ring stretching and C=O stretching |
| 1605 | C=C stretching | ||
| 1545 | 1546 | aromatic C=C stretching | |
| 1515 | 1517 | aromatic skeletal | |
| 14,351,416 | 1445 | 14,431,414 | symmetric aromatic ring stretching vibration (C=C ring);aromatic skeletal combined with C−H in-plane deformation and stretching |
| 1367 | 1344 | 1371 | aliphatic C−H stretching in methyl and phenol OH |
| 1308 | 1321 | C−H vibration of the methyl group | |
| 1240 | 1238 | 1236 | aromatic ring−O−aromatic ring stretching |
| 1201 | present in hemicelluloses | ||
| 1162 | 1145 | 1152 | C-O-C asymmetric stretching in cellulose I and cellulose II |
| 1103 | in-plane =C−H bending/C=C stretching | ||
| 1030 | 1034 | 1033 | C–O stretching/O−H out plane bending |
| 896 | 919 | β-glycosidic linkages (glucose units of cellulose chains) |
| RT (min) | Peak Area (%) | Assignment | Qual |
|---|---|---|---|
| 5.3273 | 1.6888 | 2-Furancarboxaldehyde, 5-methyl- | 93 |
| 6.5084 | 0.3951 | Piperazine, 1,4-dimethyl- | 52 |
| 6.5618 | 4.4740 | Benzeneacetaldehyde | 93 |
| 6.7815 | 0.3599 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | 62 |
| 6.9951 | 0.3635 | Thiazole | 43 |
| 7.3572 | 0.4867 | Cyclopropanecarboxylic acid, 1-amino- | 59 |
| 8.1110 | 1.7736 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 91 |
| 8.4077 | 0.2368 | Benzoic acid | 55 |
| 9.1615 | 2.5799 | Benzofuran, 2,3-dihydro- | 83 |
| 10.5563 | 2.3649 | 2-Methoxy-4-vinylphenol | 95 |
| 11.1439 | 0.6006 | Methyl 3-methoxyamino-propanoate | 38 |
| 12.1826 | 8.4919 | 2,1,3-Benzothiadiazole/2-trifluoromethyl imidazole | 53 |
| 12.2538 | 0.7550 | 2,2′-Bipyridine | 92 |
| 13.0669 | 2.7909 | 3,4-Altrosan | 49 |
| 13.6189 | 0.1442 | 1-Pyrrolidinyloxy, 3-amino-2,2,5,5-tetramethyl- | 53 |
| 13.7376 | 1.3464 | 3-Hydroxy-4-methoxybenzoic acid | 95 |
| 13.8029 | 0.2216 | 3-Piperidinone, 1,6-dimethyl- | 64 |
| 14.1590 | 5.8153 | β-D-Glucopyranoside, methyl | 58 |
| 15.7378 | 0.5604 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | 46 |
| 15.7912 | 1.5928 | 2-Propenoic acid, 3-(4-hydroxyphenyl)-, methyl ester | 98 |
| 15.8625 | 1.0835 | Tetradecanoic acid | 98 |
| 16.5450 | 0.8155 | Benzoic acid, 4-hydroxy-3,5-dimethoxy- | 98 |
| 16.7884 | 0.5167 | 2-Propenoic, 3-(4-hydroxy-3-methoxyphenyl)-, methyl ester | 99 |
| 16.9071 | 0.5716 | Pentadecanoic acid | 96 |
| 17.0851 | 1.0764 | 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)- | 94 |
| 17.1326 | 0.4642 | 2-Tetradecene, (E)- | 90 |
| 17.4175 | 4.0260 | 1,5-Heptadiene, 3,3,6-trimethyl- | 38 |
| 17.5896 | 0.9559 | Pentadecanoic acid, 14-methyl-, methyl ester | 97 |
| 17.7262 | 1.1349 | 5-Undecene | 46 |
| 17.9576 | 18.0487 | n-Hexadecanoic acid (or palmitic acid) | 99 |
| 18.3019 | 5.3442 | 3-(3,4-Dihydroxy-phenyl)-acrylic acid ethyl ester | 91 |
| 19.2753 | 0.5834 | 11-Octadecenoic acid, methyl ester | 99 |
| 19.6195 | 14.4270 | 9-Octadecenoic acid, (E)-//Oleic acid | 99 |
| 19.8154 | 9.0166 | Octadecanoic acid (or stearic acid) | 99 |
| 20.2605 | 1.6119 | 4-Methoxybenzoic acid, 2,4,5-trichlorophenyl ester | 43 |
| 20.7769 | 0.4864 | 7-Butyl-3,4,5,6(2H)-tetrahydroazepine | 49 |
| 20.8956 | 0.5923 | Isophthalic acid, di(but-3-yn-2-yl) ester | 35 |
| 24.7179 | 0.6977 | Octabenzone | 98 |
| 25.0919 | 1.5047 | Supraene | 98 |
| Treatment | EC | F. equiseti | F. oxysporum f. sp. niveum | M. phaseolina | N. falciformis | N. keratoplastica | S. sclerotiorum |
|---|---|---|---|---|---|---|---|
| COS | EC50 | 867.8 | 455.9 | 1151.7 | 721.8 | 677.5 | 864.3 |
| EC90 | 1350.4 | 1296.4 | 1420.5 | 1130.2 | 1295.4 | 1344.8 | |
| A. maritima flower extract | EC50 | 448.0 | 387.4 | 413.2 | 463.4 | 482.2 | 13.5 |
| EC90 | 832.4 | 660.1 | 664.2 | 1053.1 | 845.1 | 235.6 | |
| Hexadecanoic acid | EC50 | 297.1 | 275.9 | 156.0 | 268.3 | 230.0 | 120.3 |
| EC90 | 422.8 | 472.8 | 278.5 | 501.8 | 346.5 | 164.0 | |
| 9-octadecenoic acid | EC50 | 213.7 | 195.8 | 213.8 | 111.7 | 46.8 | 62.8 |
| EC90 | 347.2 | 354.3 | 238.7 | 242.0 | 163.2 | 110.0 | |
| Octadecanoic acid | EC50 | 231.3 | 202.6 | 269.7 | 126.4 | 35.5 | 27.7 |
| EC90 | 552.6 | 503.3 | 385.7 | 462.6 | 214.5 | 137.2 | |
| COS–A. maritima | EC50 | 320.3 | 205.7 | 308.1 | 444.1 | 442.7 | 129.2 |
| EC90 | 461.5 | 452.4 | 482.5 | 865.2 | 683.4 | 165.9 | |
| COS– hexadecanoic acid | EC50 | 110.9 | 114.0 | 36.7 | 113.9 | 103.6 | 29.3 |
| EC90 | 210.8 | 224.6 | 136.4 | 245.8 | 168.7 | 61.5 | |
| COS– 9-octadecenoic acid | EC50 | 121.5 | 107.8 | 83.2 | 79.5 | 29.7 | 21.1 |
| EC90 | 199.5 | 218.9 | 127.8 | 91.0 | 74.8 | 62.4 | |
| COS– octadecanoic acid | EC50 | 109.3 | 102.8 | 131.1 | 86.8 | 9.3 | 25.4 |
| EC90 | 256.3 | 231.4 | 193.1 | 101.9 | 48.9 | 61.2 |
| Treatment | EC | F. equiseti | F. oxysporum f. sp. niveum | M. phaseolina | N. falciformis | N. keratoplastica | S. sclerotiorum |
|---|---|---|---|---|---|---|---|
| COS– A. maritima | EC50 | 1.84 | 2.04 | 1.97 | 1.27 | 1.27 | 2.34 |
| EC90 | 2.34 | 1.93 | 1.88 | 1.36 | 1.50 | 2.42 | |
| COS– hexadecanoic acid | EC50 | 3.99 | 3.02 | 7.49 | 3.43 | 3.31 | 7.21 |
| EC90 | 3.26 | 3.09 | 3.41 | 2.96 | 3.24 | 4.75 | |
| COS– 9-octadecenoic acid | EC50 | 2.82 | 2.54 | 4.33 | 2.43 | 2.95 | 5.55 |
| EC90 | 2.84 | 2.54 | 3.20 | 4.50 | 3.88 | 3.26 | |
| COS– octadecanoic acid | EC50 | 3.34 | 2.73 | 3.33 | 2.43 | 7.25 | 2.11 |
| EC90 | 3.17 | 3.13 | 3.14 | 4.50 | 7.53 | 4.07 |
| Commercial Fungicide | Pathogen | Radial Growth of Mycelium (mm) | Inhibition (%) | |||
|---|---|---|---|---|---|---|
| Control (PDA) | Rd/10 | Rd * | Rd/10 | Rd * | ||
| Azoxystrobin | F. equiseti | 75.0 | 50.0 | 46.7 | 33.3 | 37.8 |
| F. oxysporum f. sp. niveum | 75.0 | 45.0 | 40.0 | 40.0 | 46.7 | |
| M. phaseolina | 75.0 | 38.3 | 16.7 | 48.9 | 77.8 | |
| N. falciformis | 75.0 | 43.3 | 28.3 | 42.2 | 62.2 | |
| N. keratoplastica | 75.0 | 10.0 | 0.0 | 86.7 | 100.0 | |
| S. sclerotiorum | 75.0 | 14.0 | 9.0 | 81.3 | 88.0 | |
| Mancozeb | F. equiseti | 75.0 | 70.0 | 25.0 | 6.7 | 66.7 |
| F. oxysporum f. sp. niveum | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| M. phaseolina | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| N. falciformis | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| N. keratoplastica | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| S. sclerotiorum | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Fosetyl-Al | F. equiseti | 75.0 | 75.0 | 30.0 | 0.0 | 20.0 |
| F. oxysporum f. sp. niveum | 75.0 | 66.7 | 0.0 | 11.1 | 100.0 | |
| M. phaseolina | 75.0 | 75.0 | 0.0 | 0.0 | 100.0 | |
| N. falciformis | 75.0 | 61.7 | 0.0 | 17.8 | 100.0 | |
| N. keratoplastica | 75.0 | 66.7 | 0.0 | 11.1 | 100.0 | |
| S. sclerotiorum | 75.0 | 75.0 | 13.3 | 0.0 | 82.2 | |
| Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|
| C− | 1.01 ± 0.00 a | 1.00 ± 0.00 a | 0.99 ± 0.00 ab | 0.96 ± 0.00 ab | 0.91 ± 0.00 ab | 0.89 ± 0.01 a | 0.85 ± 0.01 a |
| C+ | 1.01 ± 0.01 a | 1.00 ± 0.01 a | 0.93 ± 0.01 b | 0.91 ± 0.01 b | 0.87 ± 0.02 b | 0.59 ± 0.04 b | 0.43 ± 0.05 b |
| MIC | 1.01 ± 0.00 a | 1.00 ± 0.00 a | 0.99 ± 0.00 a | 0.96 ± 0.00 ab | 0.91 ± 0.01 ab | 0.90 ± 0.01 a | 0.84 ± 0.01 a |
| MIC×2 | 1.02 ± 0.03 a | 1.01 ± 0.03 a | 1.00 ± 0.03 a | 0.97 ± 0.03 a | 0.92 ± 0.03 ab | 0.92 ± 0.03 a | 0.82 ± 0.10 a |
| MIC×4 | 1.03 ± 0.04 a | 1.03 ± 0.04 a | 1.02 ± 0.04 a | 0.98 ± 0.04 a | 0.94 ± 0.04 a | 0.93 ± 0.04 a | 0.93 ± 0.08 a |
| Pathogen | Source/Extraction Medium | Plant | Efficacy | Ref. |
|---|---|---|---|---|
| F. equiseti | Aqueous ammonia | Tamarix gallica bark | MIC = 750 µg·mL−1 | [69] |
| Commercial essential oil | Zataria multiflora | MIC = 99–145 µg·mL−1 | [70] | |
| Heracleum persicum | MIC = 795–1180 µg·mL−1 | |||
| Pinaceae | MIC = 163–176 µg·mL−1 | |||
| Cuminum cyminum | MIC = 75–99 µg·mL−1 | |||
| Foeniculum vulgare | MIC = 63–69 µg·mL−1 | |||
| Oil cake extracts at 1–3% | Brassica napus | IR = 43.6–59.1% | [71] | |
| Cocos nucifera | IR = 7.6–22.4% | |||
| Sesame indicum | IR = 49.4–56.1% | |||
| Glycine max | IR = 0.4–5.9% | |||
| Essential oil | Piper auritum aerial parts | MIC50 = 9000 µg·mL−1 | [72] | |
| Ethanol extract | Emblica officinalis fruits | IZ = 9.5 mm | [73] | |
| Acetone extract | IZ = 10 mm | |||
| Ethanol extract | Plumbago zeylanica roots | MIC = 250 µg·mL−1 | [74] | |
| Aqueous extract at 25% | Acacia nilotica leaves | IR = 67% | [75] | |
| Achras zapota leaves | IR = 44.8% | |||
| Datura stramonium leaves | IR = 87.3% | |||
| E. officinalis leaves | IR = 75.8% | |||
| Eucalyptus globulus leaves | IR = 62.0% | |||
| Lawsonia inermis leaves | IR = 78.3% | |||
| Mimusops elengi leaves | IR = 85.8% | |||
| Peltophorum pterocarpum leaves | IR = 74.3% | |||
| Polyalthia longifolia leaves | IR = 40.5% | |||
| Prosopis juliflora leaves | IR = 76.8% | |||
| Punica granatum leaves | IR = 77.5% | |||
| Syzygium cumini leaves | IR = 68.8% | |||
| Aqueous extract | Filipendula spp. flowers | IR = 100% | [76] | |
| Allium sativum | IR = 92.2% | |||
| F. oxysporum spp. | Aqueous extract at 5, 10, and 20% | Azadirachta indica leaves | n.a. | [77] |
| Parthenium hysterophorus leaves + flowers | IR = 2.6–15.9% | |||
| Momordica charantia leaves | IR = 14.4–24.4% | |||
| A. sativum cloves | IR = 52.6–63.3% | |||
| Eucalyptus globules leaves | IR = 34.3–61.8% | |||
| Calotropis procera leaves | n.a. | |||
| Aloe vera leaves | IR = 16.6% | |||
| Beta vulgaris root | IR = 6.3–10.3% | |||
| D. stramonium leaves | IR = 61.1% | |||
| Aqueous extract at 1% | P. granatum fruits | IR = 78% | [78] | |
| Propanol extract at 1% | IR = 62% | |||
| Hexane extract | Cestrum nocturnum flowers | MIC = 1000 µg·mL−1 | [79] | |
| Chloroform extract | MIC = 1000 µg·mL−1 | |||
| Ethyl acetate extract | MIC = 500 µg·mL−1 | |||
| Methanol extract | MIC = 500 µg·mL−1 | |||
| Crude extract at 5, 10, and 20% | A. indica leaves | IR = 24.1–62.0% | [80] | |
| Ocimum sanctum leaves | IR = 7.0–17.0% | |||
| Datura metel leaves | IR = 10.1–34.2% | |||
| Cassia alata leaves | IR = 46.8–74.7% | |||
| Asparagus racemosus roots | IR = 44.3–57.0% | |||
| A. sativum bulbs | IR = 17.6–34.2% | |||
| Zingiber officinale tubers | IR = 23.7–39.5% | |||
| Ethanol extract | Flourensia microphylla leaves | MIC = 1500 µL·L−1 | [81] | |
| F. cernua leaves | MIC = 1500 µL·L−1 | |||
| F. retinophylla leaves | MIC = 1500 µL·L−1 | |||
| Aqueous extract at 5–50% | Moringa oleifera leaves | IR = 43.4–100% | [82] | |
| M. oleifera roots | IR = 48.8–100% | |||
| M. oleifera pud coats | IR = 36–100% | |||
| Commercial essential oil | Z. multiflora | MIC = 77–183 µg·mL−1 | [70] | |
| H. persicum | MIC = 753–2250 µg·mL−1 | |||
| Pinaceae | MIC = 113–147 µg·mL−1 | |||
| C. cyminum | MIC = 70–145 µg·mL−1 | |||
| F. vulgare | MIC = 77–94 µg·mL−1 | |||
| Essential oil | P. auritum aerial parts | MIC50 = 6000–9000 µg·mL−1 | [72] | |
| Aqueous extract at 25% | A. nilotica leaves | IR = 82% | [75] | |
| A. zapota leaves | IR = 34.8% | |||
| D. stramonium leaves | IR = 67.5% | |||
| E. officinalis leaves | IR = 79.5% | |||
| E. globulus leaves | IR = 59.3% | |||
| L. inermis leaves | IR = 82.0% | |||
| M. elengi leaves | IR = 86.0% | |||
| P. pterocarpum leaves | IR = 53.3% | |||
| P. longifolia leaves | IR = 36.3% | |||
| P. juliflora leaves | IR = 80.3% | |||
| P. granatum leaves | IR = 73.8% | |||
| S. cumini leaves | IR = 69.5% | |||
| Aqueous extract | Filipendula spp. flowers | IR = 95.9% | [76] | |
| A. sativum | IR = 81.4% | |||
| Ethanolic extract | Mentha spicata | MIC = 5% | [83] | |
| Aqueous extract | A. sativum leaves | MIC = 7000 µg·mL−1 | [84] | |
| M. phaseolina | Aqueous extract at 5, 10, and 20% | A. indica leaves | n.a. | [77] |
| P. hysterophorus leaves + flowers | n.a. | |||
| M. charantia leaves | n.a. | |||
| A. sativum cloves | IR = 100% | |||
| E. globules leaves | n.a. | |||
| C. procera leaves | n.a. | |||
| A. vera leaves | n.a. | |||
| B. vulgaris root | n.a. | |||
| D. stramonium leaves | IR = n.a –57.7% | |||
| Aqueous extract at 5–50% | M. oleifera leaves | IR = 17.8–82.2% | [82] | |
| M. oleifera roots | IR = 20–87.4% | |||
| M. olifera pud coats | IR = 13.8–82.2% | |||
| Chloroform extract | Ageratum conyzoides leaves | n.a. | [85] | |
| Antigonon leptopus leaves | ||||
| Chromolaena odorata leaves | ||||
| Oxalis corniculata leaves | ||||
| Passiflora foetida leaves | ||||
| Methanol extract | A. conyzoides leaves | MIC = 1250 µg·mL−1 | ||
| A. leptopus leaves | MIC = 625 µg·mL−1 | |||
| C. odorata leaves | MIC = 2500 µg·mL−1 | |||
| O. corniculata leaves | MIC = 78 µg·mL−1 | |||
| P. foetida leaves | MIC = 1250 µg·mL−1 | |||
| Aqueous extract at 5–20% | Citrus aurantifolia leaves | IR = 75.6–96.7% | [86] | |
| Ethanol extract | E. officinalis fruits | n.a. | [73] | |
| Acetone extract | ||||
| Ethanol extract | P. zeylanica roots | MIC = 500 µg·mL−1 | [74] | |
| N. keratoplastica | Essential oil | Trachyspermum ammi seeds | n.a. | [87] |
| Essential oil | Kaempferia parviflora rhizome | IZ = 17–18 mm | [88] | |
| Essential oil | Pogostemon cablin flowers + leaves | n.a. at 500 µg·mL−1 | [89] | |
| Essential oil | Origanum vulgare subsp. hirtum | MIC = 800 µg·mL−1 | [90] | |
| S. sclerotiorum | Hexane extract | C. nocturnum flowers | MIC = 1000 µg·mL−1 | [79] |
| Chloroform extract | MIC = 500 µg·mL−1 | |||
| Ethyl acetate extract | MIC = 250 µg·mL−1 | |||
| Methanol extract | MIC = 500 µg·mL−1 | |||
| Essential oils at 1, 2.5, and 5% | Thymus vulgaris | n.a. | [91] | |
| Nigella sativa | n.a. | |||
| Origanum majorana | MIC = 2.5% | |||
| Syzygium aromaticum | MIC = 2.5% | |||
| Salvia rosmarinus | n.a. | |||
| Essential oils at 20% | Ocimum basilicum | IR = 4.1% | [92] | |
| A. sativum | IR = 28.2% | |||
| Cymbopogon citratus | IR = 9.1% | |||
| Nerium oleander | IR = 14.1% | |||
| A. indica | IR = 35.5% | |||
| Allium cepa | IR = 16.9% | |||
| Essential oil | Z. officinale | MIC = 1000 µg·mL−1 | [93] | |
| Aqueous extracts | Trachystemon orientalis leaves | MIC = 7% | [94] | |
| T. orientalis flowers | MIC = 1% | |||
| Crude extracts | Rosmarinus officinalis leaves | MIC = 10% | [95] | |
| Salvia fructicosa leaves | MIC = 20% | |||
| Ethanol extract | M. spicata | MIC = 5% | [83] | |
| Aqueous extract | A. sativum leaves | MIC = 5000 µg·mL−1 | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; Martín-Ramos, P.; Navas-Gracia, L.M.; Martín-Gil, J.; Garcés-Claver, A.; Flores-León, A.; González-García, V. Armeria maritima (Mill.) Willd. Flower Hydromethanolic Extract for Cucurbitaceae Fungal Diseases Control. Molecules 2023, 28, 3730. https://doi.org/10.3390/molecules28093730
Sánchez-Hernández E, Martín-Ramos P, Navas-Gracia LM, Martín-Gil J, Garcés-Claver A, Flores-León A, González-García V. Armeria maritima (Mill.) Willd. Flower Hydromethanolic Extract for Cucurbitaceae Fungal Diseases Control. Molecules. 2023; 28(9):3730. https://doi.org/10.3390/molecules28093730
Chicago/Turabian StyleSánchez-Hernández, Eva, Pablo Martín-Ramos, Luis Manuel Navas-Gracia, Jesús Martín-Gil, Ana Garcés-Claver, Alejandro Flores-León, and Vicente González-García. 2023. "Armeria maritima (Mill.) Willd. Flower Hydromethanolic Extract for Cucurbitaceae Fungal Diseases Control" Molecules 28, no. 9: 3730. https://doi.org/10.3390/molecules28093730
APA StyleSánchez-Hernández, E., Martín-Ramos, P., Navas-Gracia, L. M., Martín-Gil, J., Garcés-Claver, A., Flores-León, A., & González-García, V. (2023). Armeria maritima (Mill.) Willd. Flower Hydromethanolic Extract for Cucurbitaceae Fungal Diseases Control. Molecules, 28(9), 3730. https://doi.org/10.3390/molecules28093730