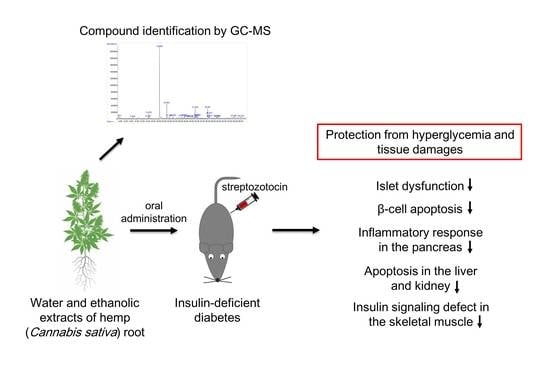
Protective Effects of Hemp (Cannabis sativa) Root Extracts against Insulin-Deficient Diabetes Mellitus In Mice
Abstract
:1. Introduction
2. Results
2.1. Hemp Root Extracts Protect Mice against STZ-Induced Hyperglycemia and Islet Dysfunction
2.2. Hemp Root Extracts Inhibit Pancreatic β-Cell Apoptosis and Cytokine-Induced Inflammatory Response
2.3. Hemp Root Extracts Attenuate Apoptosis in Liver and Kidney and Improve Insulin Signaling in Skeletal Muscle
2.4. Compound Identification in Hemp root Extracts by Gas Chromatography-Mass Spectrometry
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Animal Study
4.3. Glucose and Insulin Measurements
4.4. Histology and Immunostaining
4.5. Western Blot Analysis
4.6. Gas Chromatography-Mass Spectrometry Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khalsa, J.H.; Bunt, G.; Blum, K.; Maggirwar, S.B.; Galanter, M.; Potenza, M.N. Cannabinoids as medicinals. Curr. Addict. Rep. 2022, 9, 630–646. [Google Scholar] [CrossRef]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of cannabinoids from Cannabis sativa L.(Hemp). Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Semwogerere, F.; Katiyatiya, C.L.; Chikwanha, O.C.; Marufu, M.C.; Mapiye, C. Bioavailability and bioefficacy of hemp by-products in ruminant meat production and preservation: A review. Front. Vet. Sci. 2020, 7, 572906. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Xu, J.; Zhou, H.; Seeram, N.P.; Ma, H.; Gu, Q. Chemical constituents of industrial hemp roots and their anti-inflammatory activities. J. Cannabis Res. 2023, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Choi, J.Y.; Bae, I.A.; Kim, H.T.; Hong, S.S.; Noah, J.K.; Boo, Y.C. Identification of p-coumaric acid and ethyl p-coumarate as the main phenolic components of hemp (Cannabis sativa L.) roots. Molecules 2022, 27, 2781. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Khan, I.H.; Ferdosi, M.F. Bioactive constituents of wild Cannabis sativa roots from Pakistan. Pak. J. Weed Sci. Res. 2021, 27, 359. [Google Scholar] [CrossRef]
- Thomas, H.E.; McKenzie, M.D.; Angstetra, E.; Campbell, P.D.; Kay, T.W. Beta cell apoptosis in diabetes. Apoptosis 2009, 14, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.; Tran, P.O.T.; Poitout, V. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53, S119–S124. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, J.H.; Pan, J.H.; Kim, J.K.; Park, T.S.; Kim, Y.J.; Lee, J.H.; Kim, J.H. Naringin protects pancreatic β-cells against oxidative stress-induced apoptosis by inhibiting both intrinsic and extrinsic pathways in insulin-deficient diabetic mice. Mol. Nutr. Food. Res. 2018, 62, 1700810. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.-C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef]
- Kornpointner, C.; Martinez, A.S.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crops. Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Vuguin, P.; Saenger, P.; Dimartino-Nardi, J. Fasting glucose insulin ratio: A useful measure of insulin resistance in girls with premature adrenarche. J. Clin. Endocrinol. Metab. 2001, 86, 4618–4621. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, L.; Prause, M.; Størling, J.; Mandrup-Poulsen, T. Cytokines and pancreatic β-cell apoptosis. Adv. Clin. Chem. 2016, 75, 99–158. [Google Scholar]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef]
- Zhang, N.; Valentine, J.M.; Zhou, Y.; Li, M.E.; Zhang, Y.; Bhattacharya, A.; Walsh, M.E.; Fischer, K.E.; Austad, S.N.; Osmulski, P. Sustained NF κB inhibition improves insulin sensitivity but is detrimental to muscle health. Aging Cell 2017, 16, 847–858. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.; Jain, K.; Darah, I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem. 2008, 107, 1151–1160. [Google Scholar] [CrossRef]
- Milan Gowda, M.; Jayachandra, K.; Joshi, V.; Manjuprasanna, V.N.; Rudresha, G.V.; Velmurugan, D.; Pachaiappan, R.; Jameel, N.M.; Vishwanath, B.S. Syringol isolated from Eleusine coracana (L.) Gaertn bran suppresses inflammatory response through the down-regulation of cPLA2, COX-2, IκBα, p38 and MPO signaling in sPLA2 induced mice paw oedema. Inflammopharmacology 2022, 30, 1853–1870. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.A.; Islam, M.S. Vanillin exerts therapeutic effects against hyperglycemia-altered glucose metabolism and purinergic activities in testicular tissues of diabetic rats. Reprod. Toxicol. 2021, 102, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sharma, R.; Kar, A. Chavibetol corrects thyrotoxicosis through alterations in thyroid peroxidase. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 541–550. [Google Scholar] [CrossRef]
- Ben-Shaul, V.; Lomnitski, L.; Nyska, A.; Zurovsky, Y.; Bergman, M.; Grossman, S. The effect of natural antioxidants, NAO and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol. Lett. 2001, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Mock, N.M.; Averyanov, A.A. Redox-and bio-activity of apocynin (acetovanillone) in tobacco, a plant phenolic that alleviates symptoms of autoimmune diseases in animals. Physiol. Mol. Plant. Pathol. 2019, 106, 145–156. [Google Scholar] [CrossRef]
- Sai, B.; Issac, P.K.; Jocelyn, C.; Rakesh, J.; Chandrakumar, S.S.; Sujatha, S. An in vitro mechanistic approach towards understanding the distinct pathways regulating insulin resistance and adipogenesis by apocynin. J. Biosci. 2021, 46, 8. [Google Scholar]
- Barclay, L.R.C.; Xi, F.; Norris, J.Q. Antioxidant properties of phenolic lignin model compounds. J. Wood Chem. Technol. 1997, 17, 73–90. [Google Scholar] [CrossRef]
- Tsay, G.J.; Lin, Y.-T.; Hsu, C.-H.; Tang, F.-Y.; Kuo, Y.-H.; Chao, C.-Y. Adlay hull extracts attenuate β-amyloid-induced neurotoxicity and oxidative stress in PC12 cells through antioxidative, anti-inflammatory, and antiapoptotic activities. Biochem. Biophys. Rep. 2021, 26, 101020. [Google Scholar] [CrossRef]
- Hamed, A.B.; Mantawy, E.M.; El-Bakly, W.M.; Abdel-Mottaleb, Y.; Azab, S.S. Putative anti-inflammatory, antioxidant, and anti-apoptotic roles of the natural tissue guardian methyl palmitate against isoproterenol-induced myocardial injury in rats. Futur. J. Pharm. Sci. 2020, 6, 31. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Yagi, N.; Taguchi, K. Inhibitory compounds of α-glucosidase activity from Arctium lappa L. J. Oleo Sci. 2005, 54, 589–594. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Y.-S.; Lin, K.; Huang, X.; Chen, Y.-j.; Lai, D.; Kang, N.; Huang, L.; Weng, C.-F. A narrative review: The pharmaceutical evolution of phenolic syringaldehyde. Biomed. Pharmacother. 2022, 153, 113339. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdelmohsen, U.R. Epigenetic modifiers induce bioactive phenolic metabolites in the marine-derived fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Peng, G.; Li, L.; Na, H.; Liu, Y.; Liu, P. Palmitic acid acutely stimulates glucose uptake via activation of Akt and ERK1/2 in skeletal muscle cells. J. Lipid Res. 2011, 52, 1319–1327. [Google Scholar] [CrossRef]
- Saiki, P.; Kawano, Y.; Van Griensven, L.J.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8, 4150–4158. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Ahn, D.; Hwang, J.Y.; Kang, M.J.; Chung, S.J. Linoleic acid exerts antidiabetic effects by inhibiting protein tyrosine phosphatases associated with insulin resistance. J. Funct. Foods 2021, 83, 104532. [Google Scholar] [CrossRef]
- Collier, J.J.; Burke, S.J.; Eisenhauer, M.E.; Lu, D.; Sapp, R.C.; Frydman, C.J.; Campagna, S.R. Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS ONE 2011, 6, e22485. [Google Scholar] [CrossRef]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Størling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; Mandrup-Poulsen, T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Pirot, P.; Cardozo, A.K.; Eizirik, D.L. Mediators and mechanisms of pancreatic beta-cell death in type 1 diabetes. Arq. Bras. Endocrinol. Metabol. 2008, 52, 156–165. [Google Scholar] [CrossRef]
- Kung, C.-P.; Murphy, M.E. The role of the p53 tumor suppressor in metabolism and diabetes. J. Endocrinol. 2016, 231, R61. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chang, Y.-H.; Chiang, M.-T. Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 5795–5800. [Google Scholar] [CrossRef]
- Kelleher, A.R.; Fairchild, T.J.; Keslacy, S. STZ-induced skeletal muscle atrophy is associated with increased p65 content and downregulation of insulin pathway without NF-κB canonical cascade activation. Acta. Diabetol. 2010, 47, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wang, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F. Diabetic muscular atrophy: Molecular mechanisms and promising therapies. Front. Endocrinol. 2022, 13, 917113. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Luo, X.; Liu, Z.; Yang, L.; Lin, C.; Xu, M. Protective effect of vanillin in streptozotocin-induced diabetes in neonatal rats via attenuation of oxidative stress and inflammation. Trop. J. Phar. Res. 2019, 18, 349–355. [Google Scholar] [CrossRef]
- Sánchez-Duarte, S.; Montoya-Pérez, R.; Márquez-Gamiño, S.; Vera-Delgado, K.S.; Caudillo-Cisneros, C.; Sotelo-Barroso, F.; Sánchez-Briones, L.A.; Sánchez-Duarte, E. Apocynin attenuates diabetes-induced skeletal muscle dysfunction by mitigating ROS generation and boosting antioxidant defenses in fast-twitch and slow-twitch muscles. Life 2022, 12, 674. [Google Scholar] [CrossRef]
- Kuo, S.; Chung, H.; Huang, C.; Cheng, J. Decrease of hyperglycemia by syringaldehyde in diabetic rats. Horm. Metab. Res. 2014, 46, 8–13. [Google Scholar] [CrossRef]






| Sample | Retention Time | Compound Name | Molecular Formula | Similarity (%) Matched with Library | Peak Area (%) | Reported Biological Activities | Reference |
|---|---|---|---|---|---|---|---|
| HWE | 14.136 | Guaiacol | C7H8O2 | 87 | 2.920 | Antioxidant | [18] |
| 20.459 | 5H-1-Pyrindine | C8H7N | 81 | 1.803 | – | ||
| 20.957 | 4-Vinylguaiacol | C9H10O2 | 93 | 7.913 | Antioxidant, Anti-inflammatory | [19] | |
| 21.938 | Syringol | C8H10O3 | 97 | 3.270 | Antioxidant, Anti-inflammatory | [20,21] | |
| 23.26 | Vanillin | C8H8O3 | 91 | 0.645 | Antidiabetic, Antioxidant, Anti-inflammatory | [22,23] | |
| 24.598 | Chavibetol | C10H12O2 | 93 | 1.785 | Antioxidant | [24] | |
| 25.462 | Apocynin | C9H10O3 | 76 | 1.370 | Anti-diabetic, Antioxidant, Anti-inflammatory | [25,26,27] | |
| 26.471 | Deamino-oxo 4-methylthioamphetamine | C10H12OS | 72 | 1.652 | – | ||
| 30.505 | 2-Hydroxy-4-isopropyl-7-methoxytropone | C11H14O3 | 90 | 2.332 | – | ||
| 31.305 | Coniferyl alcohol | C10H12O3 | 89 | 4.940 | Antioxidant, Anti-inflammatory | [28,29] | |
| 35.271 | Methyl palmitate | C17H34O2 | 93 | 1.363 | Andi-diabetic, Antioxidant, Anti-inflammatory | [30,31,32] | |
| HEE | 14.137 | Mequinol | C7H8O2 | 87 | 0.925 | – | |
| 18.299 | 2,3-Dihydrobenzofuran | C8H8O | 80 | 47.141 | – | ||
| 20.953 | 4-Vinylguaiacol | C9H10O2 | 91 | 10.735 | Antioxidant, Anti-inflammatory | [19] | |
| 21.952 | Syringol | C8H10O3 | 93 | 2.056 | Antioxidant, Anti-inflammatory | [20,21] | |
| 23.265 | Vanillin | C8H8O3 | 91 | 1.805 | Antidiabetic, Antioxidant, Anti-inflammatory | [22,23] | |
| 26.477 | Deamino-oxo 4-methylthioamphetamine | C10H12OS | 72 | 1.891 | – | ||
| 29.525 | Syringaldehyde | C9H10O4 | 76 | 1.482 | Antidiabetic, Antioxidant, Anti-inflammatory | [33] | |
| 31.12 | Acetosyringone | C10H12O4 | 83 | 0.674 | Antioxidant | [34] | |
| 31.305 | Coniferyl alcohol | C10H12O3 | 96 | 6.537 | Antioxidant, Anti-inflammatory | [28,29] | |
| 35.951 | Palmitate | C16H32O2 | 95 | 7.248 | Anti-diabetic | [35] | |
| 36.601 | Ethyl palmitate | C17H34O2 | 95 | 0.894 | Anti-inflammatory | [31] | |
| 39.109 | Linoleate | C18H32O2 | 93 | 0.456 | Anti-diabetic, Anti-inflammatory | [36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, W.; Kim, S.-H.; Sim, K.-S.; Kim, K.-H.; Cho, K.-H.; Kwon, G.-S.; Lee, J.-B.; Kim, J.-H. Protective Effects of Hemp (Cannabis sativa) Root Extracts against Insulin-Deficient Diabetes Mellitus In Mice. Molecules 2023, 28, 3814. https://doi.org/10.3390/molecules28093814
Kim Y, Kim W, Kim S-H, Sim K-S, Kim K-H, Cho K-H, Kwon G-S, Lee J-B, Kim J-H. Protective Effects of Hemp (Cannabis sativa) Root Extracts against Insulin-Deficient Diabetes Mellitus In Mice. Molecules. 2023; 28(9):3814. https://doi.org/10.3390/molecules28093814
Chicago/Turabian StyleKim, Yujeong, Wonhee Kim, Soo-Hyun Kim, Kyu-Sang Sim, Ki-Hyun Kim, Kiu-Hyung Cho, Gi-Seok Kwon, Jung-Bok Lee, and Jun-Ho Kim. 2023. "Protective Effects of Hemp (Cannabis sativa) Root Extracts against Insulin-Deficient Diabetes Mellitus In Mice" Molecules 28, no. 9: 3814. https://doi.org/10.3390/molecules28093814
APA StyleKim, Y., Kim, W., Kim, S. -H., Sim, K. -S., Kim, K. -H., Cho, K. -H., Kwon, G. -S., Lee, J. -B., & Kim, J. -H. (2023). Protective Effects of Hemp (Cannabis sativa) Root Extracts against Insulin-Deficient Diabetes Mellitus In Mice. Molecules, 28(9), 3814. https://doi.org/10.3390/molecules28093814