D-Pinitol Improved Glucose Metabolism and Inhibited Bone Loss in Mice with Diabetic Osteoporosis
Abstract
:1. Introduction
2. Results
2.1. Uterus Weight
2.2. Body Weight and Food Intake
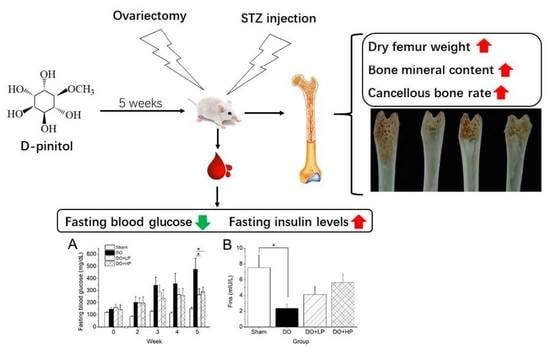
2.3. Fasting Blood Glucose Levels and Insulin Metabolism
2.4. Serum Bone Biomarkers
2.5. Bone Parameters
2.6. Inositol Content
3. Discussion
4. Material and Methods
4.1. Material
4.2. Animals
4.3. Animal Models and Treatment
4.4. Assay for Serum Biochemistry
4.5. Bone Physical Parameters and Bone Mineral Content
4.6. Inositol Content in Tissues
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cho, N.H.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Q. Trends in osteoporosis and mean bone density among type 2 diabetes patients in the US from 2005 to 2014. Sci. Rep. 2021, 11, 3693. [Google Scholar] [CrossRef] [PubMed]
- Piepkorn, B.; Kann, P.; Forst, T.; Andreas, J.; Pfützner, A.; Beyer, J. Bone mineral density and bone metabolism in diabetes mellitus. Horm. Metab. Res. 1997, 29, 584–591. [Google Scholar] [CrossRef]
- Roy, B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J. Diabetes 2013, 4, 101. [Google Scholar] [CrossRef]
- López-Ibarra, P.-J.; Pastor, M.M.C.; Escobar-Jiménez, F.; Pardo, M.D.S.; González, A.G.; Luna, J.D.D.; Requena, M.E.R.; Diosdado, M.A. Bone mineral density at time of clinical diagnosis of adult-onset type 1 diabetes mellitus. Endocr. Pract. 2001, 7, 346–351. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef]
- Sassi, F.; Buondonno, I.; Luppi, C.; Spertino, E.; Stratta, E.; Di Stefano, M.; Ravazzoli, M.; Isaia, G.; Trento, M.; Passera, P. Type 2 diabetes affects bone cells precursors and bone turnover. BMC Endocr. Disord. 2018, 18, 55. [Google Scholar] [CrossRef]
- Cushman, T.T.; Kim, N.; Hoyt, R.; Traish, A. Estradiol ameliorates diabetes-induced changes in vaginal structure of db/db mouse model. J. Sex. Med. 2009, 6, 2467–2479. [Google Scholar] [CrossRef]
- Wang, T.; Cai, L.; Wang, Y.; Wang, Q.; Lu, D.; Chen, H.; Ying, X. The protective effects of silibinin in the treatment of streptozotocin-induced diabetic osteoporosis in rats. Biomed. Pharmacother. 2017, 89, 681–688. [Google Scholar] [CrossRef]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Genistein prevents bone loss in type 2 diabetic rats induced by streptozotocin. Food Nutr. Res. 2020, 64, 3666. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Moreno, D.A.; García-Viguer, C. D-pinitol, a highly valuable product from carob pods: Health-promoting effects and metabolic pathways of this natural super-food ingredient and its derivatives. AIMS Agric. Food 2018, 3, 41–63. [Google Scholar] [CrossRef]
- Tetik, N.; Turhan, I.; Oziyci, H.R.; Karhan, M. Determination of D-pinitol in carob syrup. Int. J. Food Sci. Nutr. 2011, 62, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Park, J.-S.; Kim, M.; Hwang, B.Y.; Lee, C.-K.; Song, S. Inhibitory effect of D-pinitol on both growth and recurrence of breast tumor from MDA-MB-231 Cancer Cells. Korean J. Pharmacogn. 2014, 45, 174–180. [Google Scholar]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of D-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J. Agric. food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef]
- Geethan, P.A.; Prince, P.S.M. Antihyperlipidemic effect of D-pinitol on streptozotocin-induced diabetic wistar rats. J. Biochem. Mol. Toxicol. 2008, 22, 220–224. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subramanian, S.P. Pancreatic tissue protective nature of D-Pinitol studied in streptozotocin-mediated oxidative stress in experimental diabetic rats. Eur. J. Pharmacol. 2009, 622, 65–70. [Google Scholar] [CrossRef]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Attenuation of oxidative stress and alteration of hepatic tissue ultrastructure by D-pinitol in streptozotocin-induced diabetic rats. Free Radic. Res. 2010, 44, 668–678. [Google Scholar] [CrossRef]
- Liu, S.-C.; Chuang, S.-M.; Tang, C.-H. D-pinitol inhibits RANKL-induced osteoclastogenesis. Int. Immunopharmacol. 2012, 12, 494–500. [Google Scholar] [CrossRef]
- Kim, J.-I.; Kim, J.; Kang, M.-J.; Lee, M.-S.; Kim, J.-J.; Cha, I.-J. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: A randomized controlled study. Eur. J. Clin. Nutr. 2005, 59, 456–458. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subramanian, S.P. D-pinitol attenuates the impaired activities of hepatic key enzymes in carbohydrate metabolism of streptozotocin-induced diabetic rats. Gen. Physiol. Biophys. 2009, 28, 233–241. [Google Scholar] [CrossRef]
- Yu, J.; Choi, S.; Park, E.-S.; Shin, B.; Yu, J.; Lee, S.H.; Takami, M.; Kang, J.S.; Meong, H.; Rho, J. D-chiro-inositol negatively regulates the formation of multinucleated osteoclasts by down-regulating NFATc1. J. Clin. Immunol. 2012, 32, 1360–1371. [Google Scholar] [CrossRef]
- Davis, A.; Christiansen, M.; Horowitz, J.F.; Klein, S.; Hellerstein, M.K.; Ostlund, R.E., Jr. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 2000, 23, 1000–1005. [Google Scholar] [CrossRef]
- Silva Júnior, J.A.; Silva, A.C.D.; Figueiredo, L.S.; Araujo, T.R.; Freitas, I.N.; Carneiro, E.M.; Ribeiro, E.S.; Ribeiro, R.A. d-Pinitol increases insulin secretion and regulates hepatic lipid metabolism in Msg-obese mice. An. Acad. Bras. Ciênc. 2020, 92, e20201382. [Google Scholar] [CrossRef]
- Qi, S.; He, J.; Han, H.; Zheng, H.; Jiang, H.; Hu, C.Y.; Zhang, Z.; Li, X. Anthocyanin-rich extract from black rice (Oryza sativa L. japonica) ameliorates diabetic osteoporosis in rats. Food Funct. 2019, 10, 5350–5360. [Google Scholar] [CrossRef] [PubMed]
- Asplin, I.; Galasko, G.; Larner, J. chiro-inositol deficiency and insulin resistance: A comparison of the chiro-inositol-and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc. Natl. Acad. Sci. USA 1993, 90, 5924–5928. [Google Scholar] [CrossRef] [PubMed]
- Larner, J.; Craig, J.W. Urinary myo-inositol-to-chiro-inositol ratios and insulin resistance. Diabetes Care 1996, 19, 76–78. [Google Scholar] [CrossRef]
- Sun, T.-h.; Heimark, D.B.; Nguygen, T.; Nadler, J.L.; Larner, J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem. Biophys. Res. Commun. 2002, 293, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, D.; Navarro, J.A.; Tovar, R.; Rosell-Valle, C.; Gutiérrez-Adan, A.; Ledesma, J.C.; Sanjuan, C.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F. Activation of PI3K/Akt signaling pathway in rat hypothalamus induced by an acute oral administration of d-pinitol. Nutrients 2021, 13, 2268. [Google Scholar] [CrossRef]
- Poongothai, G.; Sripathi, S.K. A review on insulinomimetic pinitol from plants. Int. J. Pharm. Bio Sci. 2013, 4, 992–1009. [Google Scholar]
- Hamann, C.; Kirschner, S.; Günther, K.-P.; Hofbauer, L.C. Bone, sweet bone—Osteoporotic fractures in diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Onishi, T.; Wergedal, J.E.; Singer, F.; Baylink, D.J. Characterization and assay of tartrate-resistant acid phosphatase activity in serum: Potential use to assess bone resorption. Clin. Chem. 1987, 33, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Dimai, H.; Linkhart, T.; Linkhart, S.; Donahue, L.; Beamer, W.; Rosen, C.; Farley, J.; Baylink, D. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone 1998, 22, 211–216. [Google Scholar] [CrossRef]
- Guo, J.; Shi, Y.; Xu, C.; Zhong, R.; Zhang, F.; Zhang, T.; Niu, B.; Wang, J. Quantification of plasma myo-inositol using gas chromatography–mass spectrometry. Clin. Chim. Acta 2016, 460, 88–92. [Google Scholar] [CrossRef]
- Pak, Y.; Huang, L.C.; Lilley, K.J.; Larner, J. In vivo conversion of [3H] myoinositol to [3H] chiroinositol in rat tissues. J. Biol. Chem. 1992, 267, 16904–16910. [Google Scholar] [CrossRef]




| Sham | DO | DO + LP | DO + HP | |
|---|---|---|---|---|
| Ca (mM) | 2.82 ± 0.03 | 2.92 ± 0.07 | 2.91 ± 0.09 | 2.88 ± 0.04 |
| P (mM) | 2.80 ± 0.11 | 2.99 ± 0.12 | 3.52 ± 0.18 | 3.62 ± 0.29 # |
| TRAcP (U/L) | 9.32 ± 0.58 | 10.02 ± 0.89 | 9.40 ± 1.05 | 7.88 ± 0.84 |
| BALP (U/L) | 51.10 ± 4.92 | 81.04 ± 11.34 * | 88.66 ± 8.92 | 80.72 ± 12.15 |
| Sham | DO | DO + LP | DO + HP | ||
|---|---|---|---|---|---|
| Serum $ | DCI | 0.215 ± 0.020 | 0.129 ± 0.017 ** | 0.156 ± 0.020 | 0.156 ± 0.021 |
| MI | 76.889 ± 3.971 | 88.320 ± 8.445 | 77.517 ± 8.659 | 73.217 ± 3.321 | |
| DCI/MI (%) | 0.316 ± 0.014 | 0.181 ± 0.014 ** | 0.238 ± 0.026 | 0.239 ± 0.041 # | |
| Liver | DCI | 0.064 ± 0.015 | 0.030 ± 0.002 * | 0.033 ± 0.008 | 0.041 ± 0.009 |
| MI | 3.785 ± 0.464 | 3.314 ± 0.207 | 3.310 ± 0.178 | 3.763 ± 0.253 | |
| DCI/MI (%) | 1.920 ± 0.569 | 0.879 ± 0.101 * | 1.050 ± 0.296 | 1.104 ± 0.227 | |
| Kidney | DCI | 0.531 ± 0.075 | 0.178 ± 0.032 ** | 0.238 ± 0.040 | 0.471 ± 0.163 # |
| MI | 17.248 ± 1.382 | 18.102 ± 0.653 | 18.864 ± 1.003 | 18.435 ± 1.125 | |
| DCI/MI (%) | 3.083 ± 0.333 | 0.974 ± 0.160 ** | 1.282 ± 0.234 | 2.599 ± 0.911 # | |
| Bone marrow | DCI | 0.052 ± 0.009 | 0.054 ± 0.010 | 0.098 ± 0.009 | 0.167 ± 0.026 ### |
| MI | 4.290 ± 0.319 | 5.432 ± 0.459 | 4.916 ± 0.417 | 4.498 ± 0.441 | |
| DCI/MI (%) | 1.142 ± 0.214 | 0.987 ± 0.153 | 2.095 ± 0.267 # | 4.100 ± 0.898 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Koyama, T. D-Pinitol Improved Glucose Metabolism and Inhibited Bone Loss in Mice with Diabetic Osteoporosis. Molecules 2023, 28, 3870. https://doi.org/10.3390/molecules28093870
Liu X, Koyama T. D-Pinitol Improved Glucose Metabolism and Inhibited Bone Loss in Mice with Diabetic Osteoporosis. Molecules. 2023; 28(9):3870. https://doi.org/10.3390/molecules28093870
Chicago/Turabian StyleLiu, Xinxin, and Tomoyuki Koyama. 2023. "D-Pinitol Improved Glucose Metabolism and Inhibited Bone Loss in Mice with Diabetic Osteoporosis" Molecules 28, no. 9: 3870. https://doi.org/10.3390/molecules28093870
APA StyleLiu, X., & Koyama, T. (2023). D-Pinitol Improved Glucose Metabolism and Inhibited Bone Loss in Mice with Diabetic Osteoporosis. Molecules, 28(9), 3870. https://doi.org/10.3390/molecules28093870