Experimental and Theoretical Screening of Core Gold Nanoparticles and Their Binding Mechanism to an Anticancer Drug, 2-Thiouracil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Core Gold Nanoparticles (AuNPs)
2.2. Purification of Core Gold Nanoparticles (AuNPs)
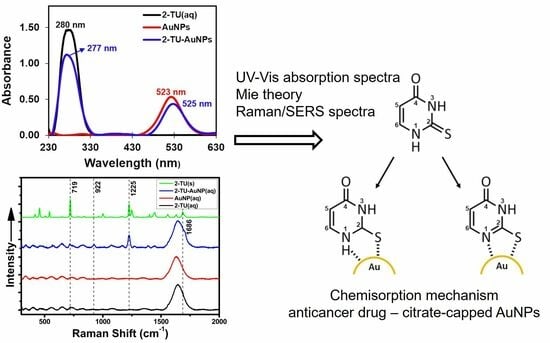
2.3. Experimental Characterization of Gold Nanoparticles Functionalized with 2-Thiouracil (2-TU-AuNPs)
2.4. Theoretical Characterization of 2-Thiouracil (2-TU) and 2-TU-Au Complexes
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Citrate-Capped AuNPs
3.3. Synthesis of Borohydride-Citrate-Capped AuNPs
3.4. Synthesis of Sodium Dodecyl Sulfate (SDS)-Capped AuNPs
3.5. Purification of AuNPs
3.6. Functionalization of AuNPs with 2-Thiouracil (2-TU)
3.7. Experimental Characterization of Unfunctionalized and Functionalized AuNPs
3.8. Theoretical Characterization of 2-TU and 2-TU-Au Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias, E. Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds. Molecules 2020, 25, 2890. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of Gold Nanoparticles in Advanced Biomedical Applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, S.; Ouyang, R.; Yang, Y.; Tao, H.; Feng, K.; Zhang, X.; Xiong, F.; Guo, N.; Zong, T.; et al. Improvement in the Anticancer Activity of 6-Mercaptopurine via Combination with Bismuth(III). Chem. Pharm. Bull. 2016, 64, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold Nanoparticles in Biological Optical Imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Benefits, Trade-Offs and Future Developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.Q.; Truong, D.H.; Nguyen, T.; Ngo, C.; Nguyen Thi, T.A.; Bui, T.H. Insight into SERS Chemical Enhancement Mechanism of Fungicide Thiram Adsorbed on Silver Nanoparticles. J. Clust. Sci. 2021, 33, 1–15. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez De Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Naveen, J.; Deshmukh, G.P.; Ravindra, M.R. Dynamic Light Scattering: Advantages and Applications. Acta Sci. Nutr. Health 2019, 3, 50–52. [Google Scholar]
- Wang, R.; Yue, L.; Yu, Y.; Zou, X.; Song, D.; Liu, K.; Liu, Y.; Su, H. Gold Nanoparticles Modify the Photophysical and Photochemical Properties of 6-Thioguanine: Preventing DNA Oxidative Damage. J. Phys. Chem. C 2016, 120, 14410–14415. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Fallah Shojaei, A.; Karimi, F.; Tabatabaeian, K.; Shakeri, S. Au Nanoparticle Loaded with 6-Thioguanine Anticancer Drug as a New Strategy for Drug Delivery. J. Nanostruct. 2018, 8, 217–424. [Google Scholar] [CrossRef]
- Li, J.-L.; Wang, L.; Liu, X.-Y.; Zhang, Z.-P.; Guo, H.-C.; Liu, W.-M.; Tang, S.-H. In Vitro Cancer Cell Imaging and Therapy Using Transferrin-Conjugated Gold Nanoparticles. Cancer Lett. 2009, 274, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lorenzana-Vázquez, G.; Pavel, I.; Meléndez, E. Gold Nanoparticles Functionalized with 2-Thiouracil for Antiproliferative and Photothermal Therapies in Breast Cancer Cells. Molecules 2023, 28, 4453. [Google Scholar] [CrossRef] [PubMed]
- Hock, N.; Racaniello, G.F.; Aspinall, S.; Denora, N.; Khutoryanskiy, V.V.; Bernkop-Schnürch, A. Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of Our Body. Adv. Sci. 2022, 9, 2102451. [Google Scholar] [CrossRef] [PubMed]
- Pragatheeswaran, A.; Kareem, T.A.; Kaliani, A.A. Effect of Plasma Exposure on Silver Nanoparticles Embedded in Polyvinyl Alcohol. J. Phys. Conf. Ser. 2010, 208, 012109. [Google Scholar] [CrossRef]
- Manikandan, D.; Mohan, S.; Nair, K.G.M. Absorption and Luminescence of Silver Nanocomposite Soda-Lime Glass Formed by Ag+–Na+ Ion-Exchange. Mater. Res. Bull. 2003, 38, 1545–1550. [Google Scholar] [CrossRef]
- Kryukov, A.I.; Stroyuk, A.L.; Zin’chuk, N.N.; Korzhak, A.V.; Kuchmii, S.Y. Optical and Catalytic Properties of Ag2S Nanoparticles. J. Mol. Catal. A Chem. 2004, 221, 209–221. [Google Scholar] [CrossRef]
- Aghevlian, S.; Yousefi, R.; Faghihi, R.; Abbaspour, A.; Niazi, A.; Jaberipour, M.; Hosseini, A. The Improvement of Anti-Proliferation Activity against Breast Cancer Cell Line of Thioguanine by Gold Nanoparticles. Med. Chem. Res. 2013, 22, 303–311. [Google Scholar] [CrossRef]
- Song, N.W.; Park, K.M.; Lee, I.-H.; Huh, H. Uncertainty Estimation of Nanoparticle Size Distribution from a Finite Number of Data Obtained by Microscopic Analysis. Metrologia 2009, 46, 480–488. [Google Scholar] [CrossRef]
- Trefry, J.C.; Monahan, J.L.; Weaver, K.M.; Meyerhoefer, A.J.; Markopolous, M.M.; Arnold, Z.S.; Wooley, D.P.; Pavel, I.E. Size Selection and Concentration of Silver Nanoparticles by Tangential Flow Ultrafiltration for SERS-Based Biosensors. J. Am. Chem. Soc. 2010, 132, 10970–10972. [Google Scholar] [CrossRef]
- Kanel, S.R.; Flory, J.; Meyerhoefer, A.; Fraley, J.L.; Sizemore, I.E.; Goltz, M.N. Influence of Natural Organic Matter on Fate and Transport of Silver Nanoparticles in Saturated Porous Media: Laboratory Experiments and Modeling. J. Nanopart Res. 2015, 17, 154. [Google Scholar] [CrossRef]
- Mabuchi, M.; Takenaka, T.; Fujiyoshi, Y.; Uyeda, N. Surface Enhanced Raman Scattering of Citrate Ions Adsorbed on Gold Sol Particles. Surf. Sci. 1982, 119, 150–158. [Google Scholar] [CrossRef]
- Ranishenka, B.V.; Panarin, A.Y.; Chelnokova, I.A.; Terekhov, S.N.; Mojzes, P.; Shmanai, V.V. Modification of a SERS-Active Ag Surface to Promote Adsorption of Charged Analytes: Effect of Cu2+ Ions. Beilstein J. Nanotechnol. 2021, 12, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Wang, K.; Zhou, Y.; Sui, C.; Wang, L.; Bai, R.; Yang, Z. Label-Free Detecting of the Compaction and Decompaction of ctDNA Molecules Induced by Surfactants with SERS Based on a nanoPAA-ZnCl2-AuLs Solid Substrate. ACS Omega 2020, 5, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, M.Y.; Borges, F.A.; Scorzoni, L.; de Lacorte Singulani, J.; Garms, B.C.; Niemeyer, J.C.; Guerra, N.B.; Pegorin Brasil, G.S.; Mussagy, C.U.; de Oliveira Carvalho, F.A.; et al. Synthesis and Characterization of Gold Nanoparticles and Their Toxicity in Alternative Methods to the Use of Mammals. J. Environ. Chem. Eng. 2021, 9, 106779. [Google Scholar] [CrossRef]
- Pore Size Chart for Different Types of Filtration. Available online: https://www.repligen.com/products/dialysis/pore-size-chart (accessed on 30 October 2023).
- Yang, W.; Hu, Y. Conformations of 2-Thiouracil in the Aqueous Solution and Its Adsorption Behavior on the Gold Substrates Explored by DFT Calculations and Experimental Methods. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 134, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, M.; Suh, J.S. Conformation of Mono- and Dicarboxylic Acids Adsorbed on Silver Surfaces. J. Am. Chem. Soc. 1985, 107, 6826–6829. [Google Scholar] [CrossRef]
- Pavel, I.; McCarney, E.; Elkhaled, A.; Morrill, A.; Plaxco, K.; Moskovits, M. Label-Free SERS Detection of Small Proteins Modified to Act as Bifunctional Linkers. J. Phys. Chem. C 2008, 112, 4880–4883. [Google Scholar] [CrossRef]
- Masoud, M.S.; Mohamed, G.B.; Abdul-Razek, Y.H.; Ali, A.E.; Khairy, F.N. Spectral, Magnetic, and Thermal Properties of Some Thiazolylazo Complexes. J. Korean Chem. Soc. 2002, 46, 99–116. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Wennmohs, F.; Neese, F. ORCA Forum. Available online: https://orcaforum.kofo.mpg.de/app.php/portal (accessed on 30 October 2023).
- Rappoport, D.; Crawford, N.R.M.; Furche, F.; Burke, K. Approximate Density Functionals: Which Should I Choose? In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-1-119-95143-8. [Google Scholar]
- Tsuzuki, S.; Uchimaru, T. Accuracy of Intermolecular Interaction Energies, Particularly Those of Hetero-Atom Containing Molecules Obtained by DFT Calculations with Grimme’s D2, D3 and D3BJ Dispersion Corrections. Phys. Chem. Chem. Phys. 2020, 22, 22508–22519. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Becker, U.; Bykov, D.; Ganyushin, D.; Hansen, A.; Izsak, R.; Liakos, D.G.; Kollmar, C.; Kossmann, S.; Pantazis, D.A.; et al. At the Max Planck Institut für Kohlenforschung. Orca Input Library. Available online: https://sites.google.com/site/orcainputlibrary/home (accessed on 30 October 2023).
- Elion, G.B.; Ide, W.S.; Hitchings, G.H. The Ultraviolet Absorption Spectra of Thiouracils1. J. Am. Chem. Soc. 1946, 68, 2137–2140. [Google Scholar] [CrossRef] [PubMed]
- Batra, K.; Zahn, S.; Heine, T. Benchmark of Simplified Time-Dependent Density Functional Theory for UV–Vis Spectral Properties of Porphyrinoids. Adv. Theory Sims. 2020, 3, 1900192. [Google Scholar] [CrossRef]
- Zhang, G.; Musgrave, C.B. Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef]
- Aihara, J. Reduced HOMO−LUMO Gap as an Index of Kinetic Stability for Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 1999, 103, 7487–7495. [Google Scholar] [CrossRef]
- Miar, M.; Shiroudi, A.; Pourshamsian, K.; Oliaey, A.R.; Hatamjafari, F. Theoretical Investigations on the HOMO–LUMO Gap and Global Reactivity Descriptor Studies, Natural Bond Orbital, and Nucleus-Independent Chemical Shifts Analyses of 3-Phenylbenzo[d]Thiazole-2(3 H)-Imine and Its Para-Substituted Derivatives: Solvent and Substituent Effects. J. Chem. Res. 2021, 45, 147–158. [Google Scholar] [CrossRef]
- American Cancer Society in Cancer Facts & Figures at Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 1 December 2023).
- He, J.; Liu, S.; Zhang, Y.; Chu, X.; Lin, Z.; Zhao, Z.; Qiu, S.; Guo, Y.; Ding, H.; Pan, Y.; et al. The Application of and Strategy for Gold Nanoparticles in Cancer Immunotherapy. Front. Pharmacol. 2021, 12, 687399. [Google Scholar] [CrossRef]
- Bromma, K.; Chithrani, D.B. Advances in Gold Nanoparticle-Based Combined Cancer Therapy. Nanomaterials 2020, 10, 1671. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzana-Vázquez, G.; Adams, D.G.; Reyna, L.G.; Meléndez, E.; Pavel, I.E. Experimental and Theoretical Screening of Core Gold Nanoparticles and Their Binding Mechanism to an Anticancer Drug, 2-Thiouracil. Molecules 2024, 29, 121. https://doi.org/10.3390/molecules29010121
Lorenzana-Vázquez G, Adams DG, Reyna LG, Meléndez E, Pavel IE. Experimental and Theoretical Screening of Core Gold Nanoparticles and Their Binding Mechanism to an Anticancer Drug, 2-Thiouracil. Molecules. 2024; 29(1):121. https://doi.org/10.3390/molecules29010121
Chicago/Turabian StyleLorenzana-Vázquez, Génesis, Daniel G. Adams, Lauren G. Reyna, Enrique Meléndez, and Ioana E. Pavel. 2024. "Experimental and Theoretical Screening of Core Gold Nanoparticles and Their Binding Mechanism to an Anticancer Drug, 2-Thiouracil" Molecules 29, no. 1: 121. https://doi.org/10.3390/molecules29010121
APA StyleLorenzana-Vázquez, G., Adams, D. G., Reyna, L. G., Meléndez, E., & Pavel, I. E. (2024). Experimental and Theoretical Screening of Core Gold Nanoparticles and Their Binding Mechanism to an Anticancer Drug, 2-Thiouracil. Molecules, 29(1), 121. https://doi.org/10.3390/molecules29010121