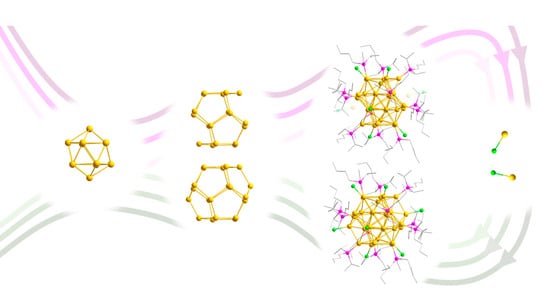
Au30(PiPr2nBu)12Cl6—An Open Cluster Provides Insight into the Influence of the Sterical Demand of the Phosphine Ligand in the Formation of Metalloid Gold Clusters
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goesmann, H.; Feldmann, C. Nanoparticulate Functional Materials. Angew. Chem. Int. Ed. 2010, 49, 1362–1395. [Google Scholar] [CrossRef] [PubMed]
- Anu Mary Ealia, S.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 32019. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Somorjai, G.A. Size and Shape Control of Metal Nanoparticles for Reaction Selectivity in Catalysis. ChemCatChem 2020, 4, 1512–1524. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- McPartlin, M.; Maso, R.; Malatesta, L. Novel Cluster Complexes of Gold(0)-Gold(I). J. Chem. Soc. D 1969, 7, 334. [Google Scholar] [CrossRef]
- Cotton, F.A. Transition-Metal compounds containing clusters of metal atoms. Q. Rev. Chem. Soc. 1966, 20, 389–401. [Google Scholar] [CrossRef]
- Purath, A.; Köppe, R.; Schnöckel, H. [Al7{N(SiMe3)2}6]–: A First Step towards Aluminum Metal Formation by Disproportionation. Angew. Chem. Int. Ed. 1999, 38, 2926–2928. [Google Scholar] [CrossRef]
- Briant, C.E.; Hall, K.P.; Mingos, D.M.P.; Wheeler, A.C. Synthesis and structural characterisation of hexakis(triphenyl phosphine)hexagold(2+) nitrate, [Au6(PPh3)6][NO3]2, and related clusters with edgesharing bitetrahedral geometries. J. Chem. Soc. Dalton Trans. 1986, 687–692. [Google Scholar] [CrossRef]
- Teo, B.K.; Shi, X.; Zhang, H. Pure gold cluster of 1:9:9:1:9:9:1 layered structure: A novel 39-metal-atom cluster [(Ph3P)14Au39Cl6]Cl2 with an interstitial gold atom in a hexagonal antiprismatic cage. J. Am. Chem. Soc. 1992, 114, 2743–2745. [Google Scholar] [CrossRef]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer–Protected Gold Nanoparticle at 1.1 Å Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, N.A.; Theivendran, S.; Ganeshraj, V.; Oliver, A.G.; Dass, A. Crystal Structure of Faradaurate-279: Au279(SPh-tBu)84 Plasmonic Nanocrystal Molecules. J. Am. Chem. Soc. 2017, 139, 15450–15459. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Shi, S.; He, R.-L.; Yuan, S.-F.; Yang, G.-Y.; Liang, G.-J.; Wang, Q.-M. Total Structure Determination of the Largest Alkynyl-Protected fcc Gold Nanocluster Au110 and the Study on Its Ultrafast Excited-State Dynamics. J. Am. Chem. Soc. 2020, 142, 18086–18092. [Google Scholar] [CrossRef] [PubMed]
- Lummis, P.A.; Osten, K.M.; Levchenko, T.I.; Sabooni Asre Hazer, M.; Malola, S.; Owens-Baird, B.; Veinot, A.J.; Albright, E.L.; Schatte, G.; Takano, S.; et al. NHC-Stabilized Au10 Nanoclusters and Their Conversion to Au25 Nanoclusters. JACS Au 2022, 2, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Strienz, M.; Fetzer, F.; Schnepf, A. Synthesis and characterisation of four bimetallic gold-gallium clusters with Au-Ga rings as a new structural motif in gold cluster chemistry. Chem. Sci. 2023, 14, 4571–4579. [Google Scholar] [CrossRef]
- Fei, W.; Antonello, S.; Dainese, T.; Dolmella, A.; Lahtinen, M.; Rissanen, K.; Venzo, A.; Maran, F. Metal Doping of Au25(SR)18- Clusters: Insights and Hindsights. J. Am. Chem. Soc. 2019, 141, 16033–16045. [Google Scholar] [CrossRef]
- Luo, X.-M.; Li, Y.-K.; Dong, X.-Y.; Zang, S.-Q. Platonic and Archimedean solids in discrete metal-containing clusters. Chem. Soc. Rev. 2023, 52, 383–444. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Kenzler, S.; Schnepf, A. Metalloid gold clusters–past, current and future aspects. Chem. Sci. 2021, 12, 3116–3129. [Google Scholar] [CrossRef]
- Adnan, R.H.; Madridejos, J.M.L.; Alotabi, A.S.; Metha, G.F.; Andersson, G.G. A Review of State of the Art in Phosphine Ligated Gold Clusters and Application in Catalysis. Adv. Sci. 2022, 9, e2105692. [Google Scholar] [CrossRef] [PubMed]
- Gutrath, B.S.; Englert, U.; Wang, Y.; Simon, U. A Missing Link in Undecagold Cluster Chemistry: Single-Crystal X-ray Analysis of [Au11(PPh3)7Cl3]. Eur. J. Inorg. Chem. 2013, 2013, 2002–2006. [Google Scholar] [CrossRef]
- Wen, F.; Englert, U.; Gutrath, B.; Simon, U. Crystal Structure, Electrochemical and Optical Properties of [Au9(PPh3)8](NO3)3. Eur. J. Inorg. Chem. 2008, 2008, 106–111. [Google Scholar] [CrossRef]
- Albano, V.G.; Bellon, P.L.; Manassero, M.; Sansoni, M. Intermetallic pattern in metal-atom clusters. Structural studies on Au11X3(PR3)7 species. J. Chem. Soc. D 1970, 1210–1211. [Google Scholar] [CrossRef]
- Kenzler, S.; Fetzer, F.; Schrenk, C.; Pollard, N.; Frojd, A.R.; Clayborne, A.Z.; Schnepf, A. Synthesis and Characterization of Three Multi-Shell Metalloid Gold Clusters Au32(R3P)12Cl8. Angew. Chem. Int. Ed. 2019, 58, 5902–5905. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhong, J.; Yang, S.; Wang, S.; Cao, T.; Zhang, J.; Li, P.; Hu, D.; Pei, Y.; Zhu, M. Crystal structure of Au₂₅(SePh)₁₈ nanoclusters and insights into their electronic, optical and catalytic properties. Nanoscale 2014, 6, 13977–13985. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-K.; Guan, Z.-J.; Wang, Q.-M. Homoleptic Alkynyl-Protected Gold Nanoclusters: Au44(PhC≡C)28 and Au36(PhC≡C)24. Angew. Chem. Int. Ed. Engl. 2017, 56, 11494–11497. [Google Scholar] [CrossRef]
- Kenzler, S.; Schrenk, C.; Schnepf, A. Au54(Et3P)18Cl12: A structurally related cluster to Au32(Et3P)12Cl8 gives insight into the formation process. Dalton Trans. 2020, 49, 10765–10771. [Google Scholar] [CrossRef]
- Fetzer, F.; Pollard, N.; Michenfelder, N.C.; Strienz, M.; Unterreiner, A.N.; Clayborne, A.Z.; Schnepf, A. Au20(tBu3P)8: A Highly Symmetric Metalloid Gold Cluster in Oxidation State 0. Angew. Chem. Int. Ed. Engl. 2022, 61, e202206019. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.-F.; Bonaccorso, T.A.; Williard, P.G.; Wang, L.-S. Controlling gold nanoclusters by diphospine ligands. J. Am. Chem. Soc. 2014, 136, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.; Theivendran, S.; Nimmala, P.R.; Kumara, C.; Jupally, V.R.; Fortunelli, A.; Sementa, L.; Barcaro, G.; Zuo, X.; Noll, B.C. Au133(SPh-tBu)52 nanomolecules: X-ray crystallography, optical, electrochemical, and theoretical analysis. J. Am. Chem. Soc. 2015, 137, 4610–4613. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Xia, N.; Liao, L.; Zhu, M.; Jin, F.; Jin, R.; Wu, Z. Unraveling the long-pursued Au144 structure by X-ray crystallography. Sci. Adv. 2018, 4, eaat7259. [Google Scholar] [CrossRef] [PubMed]
- Jover, J.; Cirera, J. Computational assessment on the Tolman cone angles for P-ligands. Dalton Trans. 2019, 48, 15036–15048. [Google Scholar] [CrossRef] [PubMed]
- Brack, M. The physics of simple metal clusters: Self-consistent jellium model and semiclassical approaches. Rev. Mod. Phys. 1993, 65, 677–732. [Google Scholar] [CrossRef]
- Van der Velden, J.W.A.; Bour, J.J.; Steggerda, J.J.; Beurskens, P.T.; Roseboom, M.; Noordik, J.H. Gold clusters. Tetrakis [1,3-bis(diphenylphosphino)propane]hexagold dinitrate: Preparation, X-ray analysis, and gold-197 Moessbauer and phosphorus-31{proton} NMR spectra. Inorg. Chem. 1982, 21, 4321–4324. [Google Scholar] [CrossRef]
- Gienger, C.; Schynowski, L.; Schaefer, J.; Schrenk, C.; Schnepf, A. New Intermetalloid Ge9-clusters with Copper and Gold: Filling Vacancies in the Cluster Chemistry of [Ge9(Hyp)3]− (Hyp=Si(SiMe3)3). Eur. J. Inorg. Chem. 2023, 26, e202200738. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement, analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Treutler, O.; Ahlrichs, R. Efficient molecular numerical integration schemes. J. Chem. Phys. 1995, 102, 346–354. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. TmoleX—A graphical user interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef]





| Bond/Distance | Au30(PiPr2nBu)12Cl6 1 | Au32(PnBu3)12Cl8 3 | Au54(PEt3)18Cl12 4 |
|---|---|---|---|
| Au-Au (inner shell) | 284.9 ± 5.7 | 285.1 ± 3.3 | 284.8 ± 10.1 |
| Au-Au (outer shell) | 314.8 ± 17.3 | 312.6 ± 11.7 | 306.0 ± 12.5 |
| Au-Au (between shells) | 278.4 ± 5.7 | 276.8 ± 3.2 | 281.5 ± 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strienz, M.; Schnepf, A. Au30(PiPr2nBu)12Cl6—An Open Cluster Provides Insight into the Influence of the Sterical Demand of the Phosphine Ligand in the Formation of Metalloid Gold Clusters. Molecules 2024, 29, 286. https://doi.org/10.3390/molecules29020286
Strienz M, Schnepf A. Au30(PiPr2nBu)12Cl6—An Open Cluster Provides Insight into the Influence of the Sterical Demand of the Phosphine Ligand in the Formation of Metalloid Gold Clusters. Molecules. 2024; 29(2):286. https://doi.org/10.3390/molecules29020286
Chicago/Turabian StyleStrienz, Markus, and Andreas Schnepf. 2024. "Au30(PiPr2nBu)12Cl6—An Open Cluster Provides Insight into the Influence of the Sterical Demand of the Phosphine Ligand in the Formation of Metalloid Gold Clusters" Molecules 29, no. 2: 286. https://doi.org/10.3390/molecules29020286
APA StyleStrienz, M., & Schnepf, A. (2024). Au30(PiPr2nBu)12Cl6—An Open Cluster Provides Insight into the Influence of the Sterical Demand of the Phosphine Ligand in the Formation of Metalloid Gold Clusters. Molecules, 29(2), 286. https://doi.org/10.3390/molecules29020286