The Effect of Block Ratio and Structure on the Thermosensitivity of Double and Triple Betaine Block Copolymers
Abstract
:1. Introduction
2. Results and Discussions
2.1. The Preparation of PGLBT-b-PSPE with Controlled Block Ratios

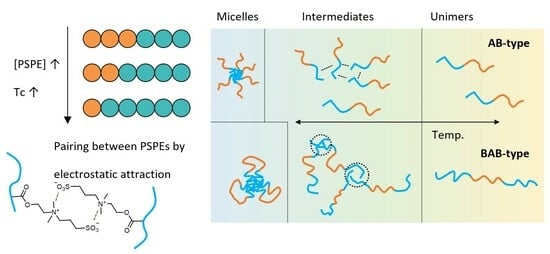
2.2. The Influence of Block Ratio on the Solution Behavior of PGLBT-b-PSPE


| Cloud Point (°C) | Rh (Unimers) (nm) | Rh (Micelles) (nm) | PDI b(Micelles) | Rg (Micelles) (nm) | Rg/Rh | |
|---|---|---|---|---|---|---|
| GLBT149-b-SPE161 | - | 4.7 | - | - | - | - |
| GLBT100-b-SPE245 | - | 4.6 | 28.3 | 0.12 | 41.7 | 1.47 |
| GLBT74-b-SPE277 | ~5 | 3.8 | 33.4 | 0.13 | 28.1 | 0.84 |
| GLBT49-b-SPE229 | 19.4 | 4.2 | (phase separation) | - | - | - |
| GLBT297-b-SPE330 | - | 6.0 | 31.2 | 0.26 | 53.4 | 1.45 |
| GLBT198-b-SPE811 | 8.4 | 4.5 | 40.2 | 0.15 | 33.9 a | 0.71 a |
| GLBT149-b-SPE742 | 14.7 | 4.6 | 44.2 | 0.11 | 40.2 a | 0.78 a |
| GLBT99-b-SPE630 | 18.7 | 3.5 | 60.9 | 0.16 | 60.4 | 0.99 |
2.3. The Solution Behavior of the BAB Triblock PSPE-b-PGLBT-b-PSPE

| Cloud Point (°C) | Rh (Unimer) (nm) | Rh (Micelles) (nm) | PDI b (Micelles) | Rg (Micelles) (nm) | Rg/Rh | |
|---|---|---|---|---|---|---|
| SPE248-GLBT99-SPE277 | 42.8 | 8.31 | 43 | 0.14 | 57.6 a | 0.87 a |
| SPE198-GLBT198-SPE212 | 12.5 | 5.20 | - | - | - | - |
3. Experimental Section
3.1. Materials
3.2. The Synthesis of PGLBT-b-PSPE via One-Pot RAFT Polymerization
3.3. Polymer Characterization
- 1H NMR spectroscopy
- Size exclusion chromatography (SEC)
- Turbidimetry
- Light scattering
- Transmission electron microscopy (TEM)
3.4. The Determination of Monomer Conversion and Theoretical Molecular Weight
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Weers, J.G.; Rathman, J.F.; Axe, F.U.; Crichlow, C.A.; Foland, L.D.; Scheuing, D.R.; Wiersema, R.J.; Zielske, A.G. Effect of the intramolecular charge separation distance on the solution properties of betaines and sulfobetaines. Langmuir 1991, 7, 854–867. [Google Scholar] [CrossRef]
- Delgado, J.D.; Schlenoff, J.B. Static and Dynamic Solution Behavior of a Polyzwitterion Using a Hofmeister Salt Series. Macromolecules 2017, 50, 4454–4464. [Google Scholar] [CrossRef]
- Mary, P.; Bendejacq, D.D.; Labeau, M.P.; Dupuis, P. Reconciling low- and high-salt solution behavior of sulfobetaine polyzwitterions. J. Phys. Chem. B 2007, 111, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4189. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.F.; Zhao, J. Understanding anti-polyelectrolyte behavior of a well-defined polyzwitterion at the single-chain level. Polym. Int. 2015, 64, 999–1005. [Google Scholar] [CrossRef]
- Matsuoka, H.; Yamakawa, Y.; Ghosh, A.; Saruwatari, Y. Nanostructure and Salt Effect of Zwitterionic Carboxybetaine Brush at the Air/Water Interface. Langmuir 2015, 31, 4827–4836. [Google Scholar] [CrossRef]
- Cao, Z.L.; Zhang, G.Z. Dynamics of polyzwitterions in salt-free and salt solutions. Phys. Chem. Chem. Phys. 2015, 17, 27045–27051. [Google Scholar] [CrossRef]
- Niu, A.Z.; Liaw, D.J.; Sang, H.C.; Wu, C. Light-scattering study of a zwitterionic polycarboxybetaine in aqueous solution. Macromolecules 2000, 33, 3492–3494. [Google Scholar] [CrossRef]
- Kathmann, E.E.L.; White, L.A.; McCormick, C.L. Water-soluble polymers. 73. Electrolyte- and pH-responsive zwitterionic copolymers of 4-(2-acrylamido-2-methylpropyl)dimethylammonio butanoate with 3-(2-acrylamido-2-methylpropyl)dimethylammonio propanesulfonate. Macromolecules 1997, 30, 5297–5304. [Google Scholar] [CrossRef]
- Kumar, R.; Fredrickson, G.H. Theory of polyzwitterion conformations. J. Chem. Phys. 2009, 131, 104901. [Google Scholar] [CrossRef]
- Azzaroni, O.; Brown, A.A.; Huck, W.T.S. UCST wetting transitions of polyzwitterionic brushes driven by self-association. Angew. Chem.-Int. Ed. 2006, 45, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Noy, J.M.; Lowe, A.B.; Roth, P.J. The synthesis and aqueous solution properties of sulfobutylbetaine (co)polymers: Comparison of synthetic routes and tuneable upper critical solution temperatures. Polym. Chem. 2015, 6, 5705–5718. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Pach, M.; Muller-Buschbaum, P.; Papadakis, C.M. Effect of the zwitterion structure on the thermo-responsive behaviour of poly(sulfobetaine methacrylates). Polym. Chem. 2017, 8, 310–322. [Google Scholar] [CrossRef]
- Laschewsky, A.; Rosenhahn, A. Molecular Design of Zwitterionic Polymer Interfaces: Searching for the Difference. Langmuir 2019, 35, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Doncom, K.E.B.; Warren, N.J.; Armes, S.P. Polysulfobetaine-based diblock copolymer nano-objects via polymerization-induced self-assembly. Polym. Chem. 2015, 6, 7264–7273. [Google Scholar] [CrossRef]
- Doncom, K.E.B.; Willcock, H.; O’Reilly, R.K. The direct synthesis of sulfobetaine-containing amphiphilic block copolymers and their self-assembly behavior. Eur. Polym. J. 2017, 87, 497–507. [Google Scholar] [CrossRef]
- Doncom, K.E.B.; Pitto-Barry, A.; Willcock, H.; Lu, A.; McKenzie, B.E.; Kirby, N.; O’Reilly, R.K. Complementary light scattering and synchrotron small-angle X-ray scattering studies of the micelle-to-unimer transition of polysulfobetaines. Soft Matter 2015, 11, 3666–3676. [Google Scholar] [CrossRef]
- Chang, Y.; Yandi, W.; Chen, W.Y.; Shih, Y.J.; Yang, C.C.; Chang, Y.; Ling, Q.-D.; Higuchi, A. Tunable Bioadhesive Copolymer Hydrogels of Thermoresponsive Poly(N-isopropyl acrylamide) Containing Zwitterionic Polysulfobetaine. Biomacromolecules 2010, 11, 1101–1110. [Google Scholar] [CrossRef]
- Yang, B.G.; Wang, C.Y.; Zhang, Y.B.; Ye, L.; Qian, Y.; Shu, Y.; Wang, J.; Li, J.; Yao, F. A thermoresponsive poly(N-vinylcaprolactam-co-sulfobetaine methacrylate) zwitterionic hydrogel exhibiting switchable anti-biofouling and cytocompatibility. Polym. Chem. 2015, 6, 3431–3442. [Google Scholar] [CrossRef]
- Dong, Z.X.; Mao, J.; Yang, M.Q.; Wang, D.P.; Bo, S.Q.; Ji, X.L. Phase Behavior of Poly(sulfobetaine methacrylate)-Grafted Silica Nanoparticles and Their Stability in Protein Solutions. Langmuir 2011, 27, 15282–15291. [Google Scholar] [CrossRef]
- Durand-Gasselin, C.; Koerin, R.; Rieger, J.; Lequeux, N.; Sanson, N. Colloidal stability of zwitterionic polymer-grafted gold nanoparticles in water. J. Colloid Interface Sci. 2014, 434, 188–194. [Google Scholar] [CrossRef]
- Virtanen, J.; Arotcarena, M.; Heise, B.; Ishaya, S.; Laschewsky, A.; Tenhu, H. Dissolution and aggregation of a poly(NIPA-block-sulfobetaine) copolymer in water and saline aqueous solutions. Langmuir 2002, 18, 5360–5365. [Google Scholar] [CrossRef]
- Vishnevetskaya, N.S.; Hildebrand, V.; Niebuur, B.J.; Grillo, I.; Filippov, S.K.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Aggregation Behavior of Doubly Thermoresponsive Polysulfobetaine-b-poly(N-isopropylacrylamide) Diblock Copolymers. Macromolecules 2016, 49, 6655–6668. [Google Scholar] [CrossRef]
- Vishnevetskaya, N.S.; Hildebrand, V.; Niebuur, B.J.; Grillo, I.; Filippov, S.K.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. “Schizophrenic” Micelles from Doubly Thermoresponsive Polysulfobetaine-b-poly(N-isopropylmethacrylamide) Diblock Copolymers. Macromolecules 2017, 50, 3985–3999. [Google Scholar] [CrossRef]
- Ranka, M.; Katepalli, H.; Blankschtein, D.; Hatton, T.A. Schizophrenic Diblock-Copolymer-Functionalized Nanoparticles as Temperature-Responsive Pickering Emulsifiers. Langmuir 2017, 33, 13326–13331. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, T.; Shao, Q.; Jiang, S.Y.; Shen, A.Q. Thermoresponsive self-assembled NiPAm-zwitterion copolymers. Polym. Chem. 2015, 6, 1066–1077. [Google Scholar] [CrossRef]
- Zhang, Z.; Vaisocherova, H.; Cheng, G.; Yang, W.; Xue, H.; Jiang, S.Y. Nonfouling Behavior of Polycarboxybetaine-Grafted Surfaces: Structural and Environmental Effects. Biomacromolecules 2008, 9, 2686–2692. [Google Scholar] [CrossRef]
- Mi, L.; Bernards, M.T.; Cheng, G.; Yu, Q.M.; Jiang, S.Y. pH responsive properties of non-fouling mixed-charge polymer brushes based on quaternary amine and carboxylic acid monomers. Biomaterials 2010, 31, 2919–2925. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S.Y. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem.-Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef]
- Birkner, M.; Ulbricht, M. Ultrafiltration membranes with markedly different pH- and ion-responsivity by photografted zwitterionic polysulfobetain or polycarbobetain. J. Membr. Sci. 2015, 494, 57–67. [Google Scholar] [CrossRef]
- Hippius, C.; Butun, V.; Erel-Goktepe, I. Bacterial anti-adhesive properties of a monolayer of zwitterionic block copolymer micelles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 41, 354–362. [Google Scholar] [CrossRef]
- Kurowska, M.; Eickenscheidt, A.; Guevara-Solarte, D.L.; Widyaya, V.T.; Marx, F.; Al-Ahmad, A.; Lienkamp, K. A Simultaneously Antimicrobial, Protein-Repellent, and Cell-Compatible Polyzwitterion Network. Biomacromolecules 2017, 18, 1373–1386. [Google Scholar] [CrossRef]
- Abraham, S.; So, A.; Unsworth, L.D. Poly(carboxybetaine methacrylamide)-Modified Nanoparticles: A Model System for Studying the Effect of Chain Chemistry on Film Properties, Adsorbed Protein Conformation, and Clot Formation Kinetics. Biomacromolecules 2011, 12, 3567–3580. [Google Scholar] [CrossRef]
- Abraham, S.; Bahniuk, M.S.; Unsworth, L.D. Plasma Protein Adsorption to Zwitterionic Poly (Carboxybetaine Methacrylate) Modified Surfaces: Chain Chemistry and End-Group Effects on Protein Adsorption Kinetics, Adsorbed Amounts and Immunoblots. Biointerphases 2012, 7, 40. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Bendinger, B.; Rijnaarts, H.H.M.; Altendorf, K.; Zehnder, A.J.B. Physicochemical cell-surface and adhesive properties of coryneform bacteria related to the presence and chain-length of mycolic acids. Appl. Environ. Microbiol. 1993, 59, 3973–3977. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappinscott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Leng, C.; Hung, H.C.; Sun, S.W.; Wang, D.; Li, Y.; Jiang, S.; Chen, Z. Probing the Surface Hydration of Nonfouling Zwitterionic and PEG Materials in Contact with Proteins. ACS Appl. Mater. Interfaces 2015, 7, 16881–16888. [Google Scholar] [CrossRef]
- Leng, C.; Sun, S.W.; Zhang, K.X.; Jiang, S.Y.; Chen, Z. Molecular level studies on interfacial hydration of zwitterionic and other antifouling polymers in situ. Acta Biomater. 2016, 40, 6–15. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, Q.; Wu, S.S.; Shen, J.; Lin, S.C. Surface modification of cellulose membranes with zwitterionic polymers for resistance to protein adsorption and platelet adhesion. J. Membr. Sci. 2010, 350, 387–394. [Google Scholar] [CrossRef]
- Lim, J.; Matsuoka, H.; Yusa, S.; Saruwatari, Y. Temperature-Responsive Behavior of Double Hydrophilic Carboxy-Sulfobetaine Block Copolymers and Their Self-Assemblies in Water. Langmuir 2019, 35, 1571–1582. [Google Scholar] [CrossRef]
- Takahashi, M.; Shimizu, A.; Yusa, S.I.; Higaki, Y. Lyotropic Morphology Transition of Double Zwitterionic Diblock Copolymer Aqueous Solutions. Macromol. Chem. Phys. 2021, 222, 2000377. [Google Scholar] [CrossRef]
- Shimizu, A.; Hifumi, E.; Kojio, K.; Takahara, A.; Higaki, Y. Modulation of Double Zwitterionic Block Copolymer Aggregates by Zwitterion-Specific Interactions. Langmuir 2021, 37, 14760–14766. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Rapid and quantitative one-pot synthesis of sequence-controlled polymers by radical polymerization. Nat. Commun. 2013, 4, 42505. [Google Scholar] [CrossRef]
- Lim, J.; Matsuoka, H.; Saruwatari, Y. One-pot synthesis of double and triple polybetaine block copolymers and their temperature-responsive solution behavior. Colloid Polym. Sci. 2021, 299, 1–13. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Agent Design and Synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Hill, M.R.; Carmean, R.N.; Sumerlin, B.S. Expanding the Scope of RAFT Polymerization: Recent Advances and New Horizons. Macromolecules 2015, 48, 5459–5469. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Exploitation of the Degenerative Transfer Mechanism in RAFT Polymerization for Synthesis of Polymer of High Livingness at Full Monomer Conversion. Macromolecules 2014, 47, 639–649. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Pushing the Limit of the RAFT Process: Multiblock Copolymers by One-Pot Rapid Multiple Chain Extensions at Full Monomer Conversion. Macromolecules 2014, 47, 3451–3460. [Google Scholar] [CrossRef]
- Gody, G.; Barbey, R.; Danial, M.; Perrier, S. Ultrafast RAFT polymerization: Multiblock copolymers within minutes. Polym. Chem. 2015, 6, 1502–1511. [Google Scholar] [CrossRef]
- Valdebenito, A.; Encinas, M.V. Effect of solvent on the free radical polymerization of N,N-dimethylacrylamide. Polym. Int. 2010, 59, 1246–1251. [Google Scholar] [CrossRef]
- Moad, G. Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization—Status of the Dilemma. Macromol. Chem. Phys. 2014, 215, 9–26. [Google Scholar] [CrossRef]
- Patterson, J.P.; Robin, M.P.; Chassenieux, C.; Colombani, O.; O’Reilly, R.K. The analysis of solution self-assembled polymeric nanomaterials. Chem. Soc. Rev. 2014, 43, 2412–2425. [Google Scholar] [CrossRef]
- Wan, W.M.; Sun, X.L.; Pan, C.Y. Morphology Transition in RAFT Polymerization for Formation of Vesicular Morphologies in One Pot. Macromolecules 2009, 42, 4950–4952. [Google Scholar] [CrossRef]
- Donovan, M.S.; Lowe, A.B.; Sanford, T.A.; McCormick, C.L. Sulfobetaine-containing diblock and triblock copolymers via reversible addition-fragmentation chain transfer polymerization in aqueous media. J. Polym. Sci. Part A-Polym. Chem. 2003, 41, 1262–1281. [Google Scholar] [CrossRef]
- Jones, E.R.; Semsarilar, M.; Blanazs, A.; Armes, S.P. Efficient Synthesis of Amine-Functional Diblock Copolymer Nanoparticles via RAFT Dispersion Polymerization of Benzyl Methacrylate in Alcoholic Media. Macromolecules 2012, 45, 5091–5098. [Google Scholar] [CrossRef]






| Mntheo (g/mol) | MnSEC (g/mol) | Đ (Mw/Mn) | |
|---|---|---|---|
| GLBT149-b-SPE161 | 77,400 | 36,400 | 1.12 |
| GLBT100-b-SPE245 | 90,300 | 43,800 | 1.17 |
| GLBT74-b-SPE277 | 93,600 | 45,100 | 1.14 |
| GLBT49-b-SPE229 | 74,900 | 41,700 | 1.15 |
| GLBT297-b-SPE330 | 156,400 | 71,300 | 1.20 |
| GLBT198-b-SPE811 | 269,500 | 89,100 | 1.18 |
| GLBT149-b-SPE742 | 239,700 | 88,300 | 1.21 |
| GLBT99-b-SPE630 | 197,600 | 66,500 | 1.16 |
| Mntheo (g/mol) | MnSEC (g/mol) | Đ (Mw/Mn) | |
|---|---|---|---|
| SPE248-GLBT99-SPE277 | 168,300 | 66,300 | 1.20 |
| SPE198-GLBT198-SPE212 | 157,500 | 64,500 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Matsuoka, H.; Kinoshita, Y.; Yusa, S.-i.; Saruwatari, Y. The Effect of Block Ratio and Structure on the Thermosensitivity of Double and Triple Betaine Block Copolymers. Molecules 2024, 29, 390. https://doi.org/10.3390/molecules29020390
Lim J, Matsuoka H, Kinoshita Y, Yusa S-i, Saruwatari Y. The Effect of Block Ratio and Structure on the Thermosensitivity of Double and Triple Betaine Block Copolymers. Molecules. 2024; 29(2):390. https://doi.org/10.3390/molecules29020390
Chicago/Turabian StyleLim, Jongmin, Hideki Matsuoka, Yusuke Kinoshita, Shin-ichi Yusa, and Yoshiyuki Saruwatari. 2024. "The Effect of Block Ratio and Structure on the Thermosensitivity of Double and Triple Betaine Block Copolymers" Molecules 29, no. 2: 390. https://doi.org/10.3390/molecules29020390
APA StyleLim, J., Matsuoka, H., Kinoshita, Y., Yusa, S. -i., & Saruwatari, Y. (2024). The Effect of Block Ratio and Structure on the Thermosensitivity of Double and Triple Betaine Block Copolymers. Molecules, 29(2), 390. https://doi.org/10.3390/molecules29020390