Core-Hole Excitation Spectra of the Oxides and Hydrates of Fullerene C60 and Azafullerene C59N
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carbon K-edge NEXAFS and XES
2.2. Nitrogen K-edge NEXAFS and XES
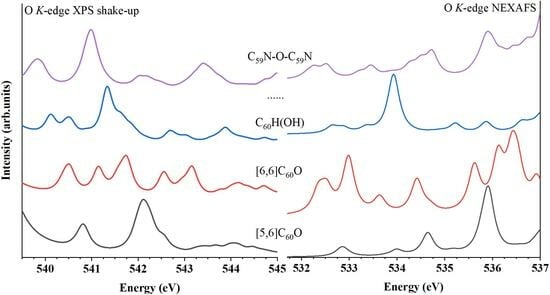
2.3. Oxygen K-edge NEXAFS and XES
2.4. XPS Shake-Up Satellites
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nam, S.; Khim, D.; Martinez, G.T.; Varambhia, A.; Nellist, P.D.; Kim, Y.; Anthopoulos, T.D.; Bradley, D.D. Significant Performance Improvement in n-Channel Organic Field-Effect Transistors with C60:C70 Co-Crystals Induced by Poly (2-ethyl-2-oxazoline) Nanodots. Adv. Mater. 2021, 33, 2100421. [Google Scholar] [CrossRef] [PubMed]
- Benatto, L.; Marchiori, C.; Talka, T.; Aramini, M.; Yamamoto, N.; Huotari, S.; Roman, L.; Koehler, M. Comparing C60 and C70 as acceptor in organic solar cells: Influence of the electronic structure and aggregation size on the photovoltaic characteristics. Thin Solid Films 2020, 697, 137827. [Google Scholar] [CrossRef]
- Alipour, E.; Alimohammady, F.; Yumashev, A.; Maseleno, A. Fullerene C60 containing porphyrin-like metal center as drug delivery system for ibuprofen drug. J. Mol. Model. 2020, 26, 7. [Google Scholar] [CrossRef]
- Hong, I.-H.; Gao, C.-J.; Lin, K.-B.; Kaun, C.-C. Self-organized C70/C60 heterojunction nanowire arrays on Si(110) for Si-based molecular negative differential resistance nanodevices. Appl. Surf. Sci. 2020, 531, 147338. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Kumar, S. Thermal transport in fullerene derivatives using molecular dynamics simulations. Sci. Rep. 2015, 5, 12763. [Google Scholar] [CrossRef] [PubMed]
- Socol, M.; Preda, N.; Costas, A.; Borca, B.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G.; Stanculescu, A. Thin films based on cobalt phthalocyanine: C60 fullerene: ZnO hybrid nanocomposite obtained by laser evaporation. Nanomaterials 2020, 10, 468. [Google Scholar] [CrossRef]
- Tanuma, Y.; Knaflič, T.; Anézo, B.; Stangel, C.; Volkmann, J.; Tagmatarchis, N.; Wegner, H.A.; Arčon, D.; Ewels, C.P. Long Spin Coherence Times on C59N-C60 Heterodimer Radicals Entrapped in Cycloparaphenylene Rings. J. Phys. Chem. C 2023, 127, 6552–6561. [Google Scholar] [CrossRef]
- Martín-Gomis, L.; Rotas, G.; Ohkubo, K.; Fernández-Lázaro, F.; Fukuzumi, S.; Tagmatarchis, N.; Sastre-Santos, Á. Does a nitrogen matter? Synthesis and photoinduced electron transfer of perylenediimide donors covalently linked to C59N and C60 acceptors. Nanoscale 2015, 7, 7437–7444. [Google Scholar] [CrossRef]
- Rotas, G.; Stranius, K.; Tkachenko, N.; Tagmatarchis, N. Ultralong 20 Milliseconds Charge Separation Lifetime for Photoilluminated Oligophenylenevinylene-Azafullerene Systems. Adv. Funct. Mater. 2018, 28, 1702278. [Google Scholar] [CrossRef]
- Yin, H.; Lin, H.; Zong, Y.; Wang, X.-D. The recent advances in C60 micro/nanostructures and their optoelectronic applications. Org. Electron. 2021, 93, 106142. [Google Scholar] [CrossRef]
- Kianezhad, M.; Youzi, M.; Vaezi, M.; Nejat Pishkenari, H. Unidirectional motion of C60-based nanovehicles using hybrid substrates with temperature gradient. Sci. Rep. 2023, 13, 1100. [Google Scholar] [CrossRef]
- Vinnikov, N.; Dolbin, A.; Basnukaeva, R.; Gavrilko, V.; Eselson, V.; Buravtseva, L. Quantum effects in the low-temperature thermal expansion of fullerite C60 doped with a 4He impurity. Low Temp. Phys. 2022, 48, 791–797. [Google Scholar] [CrossRef]
- Elistratova, M.; Zakharova, I. Temperature-dependent photoluminescence of thin tetraphenylporphyrin-based thin films and their composites with C60 fullerene. J. Mater. Sci. Mater. Electron. 2022, 33, 15554–15562. [Google Scholar] [CrossRef]
- Sivkov, D.V.; Petrova, O.V.; Nekipelov, S.V.; Vinogradov, A.S.; Skandakov, R.N.; Bakina, K.A.; Isaenko, S.I.; Ob’edkov, A.M.; Kaverin, B.S.; Vilkov, I.V.; et al. Quantitative Characterization of Oxygen-Containing Groups on the Surface of Carbon Materials: XPS and NEXAFS Study. Appl. Sci. 2022, 12, 7744. [Google Scholar] [CrossRef]
- Lin, I.H.; Lu, Y.H.; Chen, H.T. Nitrogen-doped C60 as a robust catalyst for CO oxidation. J. Comput. Chem. 2017, 38, 2041–2046. [Google Scholar] [CrossRef]
- Tang, K.; Chen, X.; Ding, X.; Yu, X.; Yu, X. MoS2/graphene oxide/C60-OH nanostructures deposited on a quartz crystal microbalance transducer for humidity sensing. ACS Appl. Nano Mater. 2021, 4, 10810–10818. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Janebi, H. B-, N-doped and BN codoped C60 heterofullerenes for environmental monitoring of NO and NO2: A DFT study. Mol. Phys. 2020, 118, e1631495. [Google Scholar] [CrossRef]
- Tabari, L.; Farmanzadeh, D. Interesting adsorption behavior of C60O fullerene oxide isomers toward O3 and CO molecules: A DFT study. Appl. Surf. Sci. 2019, 479, 569–575. [Google Scholar] [CrossRef]
- Dattani, R.; Gibson, K.F.; Few, S.; Borg, A.J.; DiMaggio, P.A.; Nelson, J.; Kazarian, S.G.; Cabral, J.T. Fullerene oxidation and clustering in solution induced by light. J. Colloid Interface Sci. 2015, 446, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sanchís, J.; Freixa, A.; López-Doval, J.C.; Santos, L.H.; Sabater, S.; Barceló, D.; Abad, E.; Farré, M. Bioconcentration and bioaccumulation of C60 fullerene and C60 epoxide in biofilms and freshwater snails (Radix sp.). Environ. Res. 2020, 180, 108715. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Britz, D.A.; Morton, J.J.; Khlobystov, A.N.; Porfyrakis, K.; Ardavan, A.; Briggs, G.A.D. Synthesis and reactivity of N@C60O. Phys. Chem. Chem. Phys. 2006, 8, 2083–2088. [Google Scholar] [CrossRef]
- Mondal, T.; Tripathi, A.; Tiwari, A.; Zhang, J.; Shripathi, T.; Shinohara, H.J.J.o.A.P. Temperature and pressure induced Raman studies of C60 oxide. J. Appl. Phys. 2018, 124, 195105. [Google Scholar] [CrossRef]
- Gao, B.; Chen, G. CO oxidization catalyzed by B, N, and their co-doped fullerenes: A first-principles investigation. RSC. Adv. 2019, 9, 21626–21636. [Google Scholar] [CrossRef]
- Palotás, J.; Martens, J.; Berden, G.; Oomens, J. Laboratory IR Spectra of the Ionic Oxidized Fullerenes C60O+ and C60OH+. J. Phys. Chem. A 2022, 126, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Kelly, L.L.; Winget, P.; Li, H.; Kim, H.; Ndione, P.F.; Sigdel, A.K.; Berry, J.J.; Graham, S.; Brédas, J.L.; et al. Tailoring Electron-Transfer Barriers for Zinc Oxide/C60 Fullerene Interfaces. Adv. Funct. Mater. 2014, 24, 7381–7389. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kanai, K.; Ouchi, Y.; Seki, K. Oxygen effect on the interfacial electronic structure of C60 film studied by ultraviolet photoelectron spectroscopy. Chem. Phys. Lett. 2007, 441, 63–67. [Google Scholar] [CrossRef]
- Wang, X.-B.; Woo, H.-K.; Kiran, B.; Wang, L.-S. Photoelectron Spectroscopy and Electronic Structures of Fullerene Oxides: C60Ox− (x = 1–3). J. Phys. Chem. A 2005, 109, 11089–11092. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Park, J.; Yeom, G.Y. Interfacial electronic structure of molybdenum oxide on the fullerene layer, a potential hole-injecting layer in inverted top-emitting organic light-emitting diodes. Curr. Appl. Phys. 2013, 13, 1037–1041. [Google Scholar] [CrossRef]
- Pan, F.; Ni, K.; Xu, T.; Chen, H.; Wang, Y.; Gong, K.; Liu, C.; Li, X.; Lin, M.-L.; Li, S.; et al. Long-range ordered porous carbons produced from C60. Nature 2023, 614, 95–101. [Google Scholar] [CrossRef]
- Tropin, T.V.; Karpets, M.L.; Kosiachkin, Y.; Gapon, I.V.; Gorshkova, Y.E.; Aksenov, V.L. Evaluation of fullerenes C60/C70 layers in polystyrene thin films by neutron and X-ray reflectometry. Fuller. Nanotub. Carbon Nanostructures 2021, 29, 819–824. [Google Scholar] [CrossRef]
- Asad, K.; Stergiou, A.; Kourtellaris, A.; Tagmatarchis, N.; Chronakis, N. First Synthesis of the Inherently Chiral Trans-4′ Bisadduct of C59N Azafullerene by Using Cyclo-[2]-dodecylmalonate as a Tether. Chem. Eur. J. 2021, 27, 13879–13886. [Google Scholar] [CrossRef]
- Couto, R.C.; Kjellsson, L.; Ågren, H.; Carravetta, V.; Sorensen, S.L.; Kubin, M.; Bülow, C.; Timm, M.; Zamudio-Bayer, V.; von Issendorff, B.; et al. The carbon and oxygen K-edge NEXAFS spectra of CO+. Phys. Chem. Chem. Phys. 2020, 22, 16215–16223. [Google Scholar] [CrossRef]
- de Kock, S.; Skudler, K.; Matsidik, R.; Sommer, M.; Müller, M.; Walter, M. NEXAFS spectra of model sulfide chains: Implications for sulfur networks obtained from inverse vulcanization. Phys. Chem. Chem. Phys. 2023, 25, 20395–20404. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, T.; Ljubić, I.; Kivimäki, A.; Richter, R. Core–shell excitation of isoxazole at the C, N, and O K-edges–an experimental NEXAFS and theoretical TD-DFT study. Phys. Chem. Chem. Phys. 2022, 24, 19302–19313. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Henderson, Z.; Thomas, A.G.; Compeán-González, C.L.; Greer, A.J.; Hardacre, C.; Venturini, F.; Garzon, W.Q.; Ferrer, P.; Grinter, D.C.; et al. Near-ambient pressure XPS and NEXAFS study of a superbasic ionic liquid with CO2. J. Phys. Chem. C 2021, 125, 22778–22785. [Google Scholar] [CrossRef]
- Braglia, L.; Tavani, F.; Mauri, S.; Edla, R.; Krizmancic, D.; Tofoni, A.; Colombo, V.; D’angelo, P.; Torelli, P. Catching the Reversible Formation and Reactivity of Surface Defective Sites in Metal–Organic Frameworks: An Operando Ambient Pressure-NEXAFS Investigation. J. Phys. Chem. Lett. 2021, 12, 9182–9187. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Hawly, T.; Zhao, B.; Halik, M.; Nefedov, A.; Fink, R. Field-induced modification of the electronic structure in BTBT-based organic thin films observed by NEXAFS spectroscopy. Appl. Phys. Lett. 2022, 121, 183503. [Google Scholar] [CrossRef]
- Couto, R.C.; Hua, W.; Lindblad, R.; Kjellsson, L.; Sorensen, S.L.; Kubin, M.; Bülow, C.; Timm, M.; Zamudio-Bayer, V.; von Issendorff, B.; et al. Breaking inversion symmetry by protonation: Experimental and theoretical NEXAFS study of the diazynium ion, N2H+. Phys. Chem. Chem. Phys. 2021, 23, 17166–17176. [Google Scholar] [CrossRef] [PubMed]
- Lever, F.; Mayer, D.; Metje, J.; Alisauskas, S.; Calegari, F.; Düsterer, S.; Feifel, R.; Niebuhr, M.; Manschwetus, B.; Kuhlmann, M.; et al. Core-level spectroscopy of 2-thiouracil at the sulfur L1-and L2,3-edges utilizing a SASE free-electron laser. Molecules 2021, 26, 6469. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.P.; Hall, S.J.; Maurer, R.J. The nuts and bolts of core-hole constrained ab initio simulation for K-shell x-ray photoemission and absorption spectra. J. Phys. Condens. Matter 2021, 33, 154005. [Google Scholar] [CrossRef]
- Armillotta, F.; Bidoggia, D.; Biasin, P.; Annese, A.; Cossaro, A.; Verdini, A.; Floreano, L.; Peressi, M.; Vesselli, E. Spectroscopic fingerprints of iron-coordinated cobalt and iron porphyrin layers on graphene. Cell Rep. Phys. Sci. 2023, 4, 101378. [Google Scholar] [CrossRef]
- Creegan, K.M.; Robbins, J.L.; Robbins, W.K.; Millar, J.M.; Sherwood, R.D.; Tindall, P.J.; Cox, D.M.; McCauley, J.P.; Jones, D.R. Synthesis and characterization of C60O, the first fullerene epoxide. J. Am. Chem. Soc. 1992, 114, 1103–1105. [Google Scholar] [CrossRef]
- Lebedkin, S.; Ballenweg, S.; Gross, J.; Taylor, R.; Krätschmer, W. Synthesis of C120O: A new dimeric [60] fullerene derivative. Tetrahedron Lett. 1995, 36, 4971–4974. [Google Scholar] [CrossRef]
- Weisman, R.B.; Heymann, D.; Bachilo, S.M. Synthesis and characterization of the “missing” oxide of C60:[5,6]-open C60O. J. Am. Chem. Soc. 2001, 123, 9720–9721. [Google Scholar] [CrossRef] [PubMed]
- Erbahar, D.; Susi, T.; Rocquefelte, X.; Bittencourt, C.; Scardamaglia, M.; Blaha, P.; Guttmann, P.; Rotas, G.; Tagmatarchis, N.; Zhu, X.; et al. Spectromicroscopy of C60 and azafullerene C59N: Identifying surface adsorbed water. Sci. Rep. 2016, 6, 35605. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gao, B.; Deng, M.; Luo, Y. A comparative theoretical study on core-hole excitation spectra of azafullerene and its derivatives. J. Chem. Phys. 2014, 140, 124304. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Mohammadi, M.D.; Abdullah, H.Y.; Louis, H.; Etim, E.E.; Edet, H.O. Evaluating the detection potential of C59X fullerenes (X= C, Si, Ge, B, Al, Ga, N, P, and As) for H2SiCl2 molecule. J. Mol. Liq. 2023, 387, 122621. [Google Scholar] [CrossRef]
- Sood, P.; Kim, K.C.; Jang, S.S. Electrochemical and electronic properties of nitrogen doped fullerene and its derivatives for lithium-ion battery applications. J. Energy Chem. 2018, 27, 528–534. [Google Scholar] [CrossRef]
- Nattagh, F.; Hosseini, S.; Esrafili, M.D. Effects of B and N doping/codoping on the adsorption behavior of C60 fullerene towards aspirin: A DFT investigation. J. Mol. Liq. 2021, 342, 117459. [Google Scholar] [CrossRef]
- Käämbre, T.; Qian, L.; Rubensson, J.-E.; Guo, J.-H.; Såthe, C.; Nordgren, J.; Palmqvist, J.-P.; Jansson, U. Study of oxygen-C60 compound formation by NEXAFS and RIXS. Eur. Phys. J. D 2001, 16, 357–360. [Google Scholar] [CrossRef]
- Schulte, K.; Wang, L.; Moriarty, P.; Prassides, K.; Tagmatarchis, N. Resonant processes and Coulomb interactions in (C59N)2. J. Chem. Phys. 2007, 126, 184707. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, D.; Tian, H.-R.; Wu, Q.; Hou, S.; Pi, J.; Sadeghi, H.; Tang, Z.; Yang, Y.; Liu, J.; et al. Atomically defined angstrom-scale all-carbon junctions. Nat. Commun. 2019, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, R.; Zheng, T.; Lu, Z.; Fang, Y.; Xie, H.; Wang, W.; Xue, W. Rational Design of a Low-Dimensional and Metal-free Heterostructure for Efficient Water Oxidation: DFT and Experimental Studies. Langmuir 2022, 38, 12562–12569. [Google Scholar] [CrossRef]
- Wohlers, M.; Werner, H.; Herein, D.; Schedel-Niedrig, T.; Bauer, A.; Schlögl, R. Reaction of C60 and C70 with molecular oxygen. Synth. Met. 1996, 77, 299–302. [Google Scholar] [CrossRef]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Scalmani, G.; Barone, V.; Petersson, G.A.; Li, X.; Caricato, M.; Marenich, A.V.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Triguero, L.; Pettersson, L.; Ågren, H. Calculations of near-edge X-ray-absorption spectra of gas-phase and chemisorbed molecules by means of density-functional and transition-potential theory. Phys. Rev. B 1998, 58, 8097. [Google Scholar] [CrossRef]
- Delesma, F.A.; Van den Bossche, M.; Grönbeck, H.; Calaminici, P.; Köster, A.M.; Pettersson, L.G. A chemical view on X-ray photoelectron spectroscopy: The ESCA molecule and surface-to-bulk XPS shifts. ChemPhysChem 2018, 19, 169–174. [Google Scholar] [CrossRef]
- Hua, W.; Gao, B.; Luo, Y. First-principle simulation of soft X-ray spectroscopy. Prog. Chem. 2012, 24, 964. [Google Scholar]
- Nyberg, M.; Luo, Y.; Triguero, L.; Pettersson, L.G.; Ågren, H. Core-hole effects in x-ray-absorption spectra of fullerenes. Phys. Rev. B 1999, 60, 7956. [Google Scholar] [CrossRef]
- Bassan, A.; Nyberg, M.; Luo, Y. Identifying isomers of C78 by means of x-ray spectroscopy. Phys. Rev. B 2002, 65, 165402. [Google Scholar] [CrossRef]
- Gao, B.; Liu, L.; Wang, C.; Wu, Z.; Luo, Y. Spectral identification of fullerene C82 isomers. J. Chem. Phys. 2007, 127, 164314. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.; Luo, Y. Electronic structures of azafullerene C48N12. J. Chem. Phys. 2003, 119, 7139–7144. [Google Scholar] [CrossRef]
- Qi, J.; Hua, W.; Gao, B. Theoretical study of two Ih-symmetry-breaking C60 isomers and their chlorinated species in core-excited and ground states. Chem. Phys. Lett. 2012, 539, 222–228. [Google Scholar] [CrossRef]
- Pettersson, L.G.; Wahlgren, U.; Gropen, O. Effective core potential parameters for first-and second-row atoms. J. Chem. Phys. 1987, 86, 2176–2184. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.; Pettersson, L.; Casida, M.; Daul, C.; Goursot, A.; Koester, A.; Proynov, E.; St-Amant, A.; Salahub, D.R.; Carravetta, V.; et al. StoBe-deMon v.3.3; Royal Institute of Technology: Stockholm, Sweden; Berlin, Germany, 2014. [Google Scholar]
- Vall-Llosera, G.; Gao, B.; Kivimäki, A.; Coreno, M.; Ruiz, J.; de Simone, M.; Ågren, H.; Rachlew, E. The C 1s and N 1s near edge x-ray absorption fine structure spectra of five azabenzenes in the gas phase. J. Chem. Phys. 2008, 128, 044316. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, J.; Liu, K.; Luo, Y. Bionano-Lego, Version 2.0; Royal Institute of Technology: Stockholm, Sweden, 2008. [Google Scholar]
- Brena, B.; Carniato, S.; Luo, Y. Functional and basis set dependence of K-edge shake-up spectra of molecules. J. Chem. Phys. 2005, 122, 184316. [Google Scholar] [CrossRef]
- Brena, B.; Luo, Y.; Nyberg, M.; Carniato, S.; Nilson, K.; Alfredsson, Y.; Åhlund, J.; Mårtensson, N.; Siegbahn, H.; Puglia, C. Equivalent core-hole time-dependent density functional theory calculations of carbon 1s shake-up states of phthalocyanine. Phys. Rev. B 2004, 70, 195214. [Google Scholar] [CrossRef]
- Brena, B.; Luo, Y. Time-dependent DFT calculations of core electron shake-up states of metal-(free)-phthalocyanines. Radiat. Phys. Chem. 2006, 75, 1578–1581. [Google Scholar] [CrossRef]
- Gao, B.; Wu, Z.; Luo, Y. A density functional theory study of shake-up satellites in photoemission of carbon fullerenes and nanotubes. J. Chem. Phys. 2008, 128, 234704. [Google Scholar] [CrossRef]
- Kutzelnigg, W.; Fleischer, U.; Schindler, M. The IGLO-method: Ab initio calculation and interpretation of NMR chemical shifts and magnetic susceptibilities. Deuterium Shift Calc. 1990, 23, 165–262. [Google Scholar]
- Zhang, B.-B.; Lin, J.; Song, X.-N.; Wang, C.-K.; Hua, W.; Ma, Y. Identification of oxidation states in γ-graphyne by computational XPS and NEXAFS spectra. Appl. Surf. Sci. 2023, 609, 155134. [Google Scholar] [CrossRef]
- Ming, J.; Zhang, J.-R.; Song, X.-N.; Li, X.; Hua, W.; Ma, Y. First-principles simulation of X-ray spectra of graphdiyne and graphdiyne oxides at the carbon K-edge. Phys. Chem. Chem. Phys. 2023, 25, 32421–32429. [Google Scholar] [CrossRef] [PubMed]





| Molecule | HOMO Energy (eV) | LUMO Energy (eV) | Egap (eV) | BE (kcal/mol) | Sym. | Number of Symmetrically Inequivalent Carbon |
|---|---|---|---|---|---|---|
| C60 | −5.99 | −3.23 | 2.76 | – | Ih | 1 |
| [5,6]C60O | −5.90 | −3.30 | 2.60 | −78.92 | Cs | 32 |
| [6,6]C60O | −5.94 | −3.33 | 2.61 | −76.81 | C2v | 16 |
| C60H(OH) | −5.75 | −3.19 | 2.56 | −122.22 | Cs | 32 |
| C60-O-C60 | −5.77 | −3.31 | 2.46 | −94.02 | C2v | 32 |
| C60H-O-C60H | −5.71 | −3.27 | 2.44 | −220.17 | C2v | 32 |
| C59N | −4.58 (α spin) −5.94 (β spin) | −3.29 (α spin) −3.46 (β spin) | 1.29 (α spin) 2.48 (β spin) | – | Cs | 31 |
| C59N(OH) | −5.62 | −3.24 | 2.38 | −72.53 | Cs | 31 |
| (C59N)2 | −5.52 | −3.29 | 2.23 | −31.81 | C2h | 31 |
| C59N-O-C59N | −5.55 | −3.33 | 2.22 | −123.60 | C2v | 31 |
| Molecule | Peak (Shoulder) A (eV) (1s → LUMO) | Peak B (eV) | Peak C (eV) |
|---|---|---|---|
| [5,6]C60O | 532.80 | − | − |
| [6,6]C60O | 532.35 | 532.98 | − |
| C60H(OH) | 532.64 | 533.93 | − |
| C60-O-C60 | 532.03 | 532.64 | − |
| C60H-O-C60H | 531.97 | 533.00 | − |
| C59N(OH) | 532.78 | 533.82 | 532.97 |
| C59N-O-C59N | 532.04 | 532.51 | 533.45 |
| Molecule | A | C |
|---|---|---|
| [5,6]C60O | C1: 291.284 eV | … a |
| [6,6]C60O | - | C2: 292.035 eV |
| C60H(OH) | C4: 290.888 eV | C3: 292.257 eV |
| C60-O-C60 | C3: 291.055 eV | C4: 292.064 eV |
| C60H-O-C60H | C4: 290.735 eV | C3: 292.114 eV |
| C59N | C5: 291.245 eV (α spin) | … a |
| C5: 291.228 eV (β spin) | ||
| C59N(OH) | C5: 291.096 eV | … a |
| (C59N)2 | C5: 290.989 eV | C3: 292.003 eV |
| C59N-O-C59N | C5: 290.952 eV | … a |
| Molecule | sp2-like Carbon Atom (Blue-Shift) | sp3-like Carbon Atom (Blue-Shift) |
|---|---|---|
| [5,6]C60O | C1 (∼1 eV) | - |
| [6,6]C60O | - | C2 (∼2 eV) |
| C60H(OH) | - | C3 (∼2 eV) |
| - | C4 (∼1 eV) | |
| C60-O-C60 | - | C3 (∼1 eV) |
| - | C4 (∼2 eV) | |
| C60H-O-C60H | - | C3 (∼2 eV) |
| - | C4 (∼1 eV) | |
| C59N | C3 (–) | - |
| C5 (∼1 eV) | - | |
| C59N(OH) | C5 (∼1 eV) | C3 (∼3 eV) |
| (C59N)2 | C5 (∼1 eV) | C3 (∼2 eV) |
| C59N-O-C59N | C5 (∼1 eV) | C3 (∼3 eV) |
| Molecule | Energy of Peak B (eV) |
|---|---|
| C59N | 409.19 |
| C59N(OH) | 409.31 |
| (C59N)2 | 409.34 |
| C59N-O-C59N | 409.46 |
| Molecule | Energy of Peak A (eV) | Energy of Peak (Shoulder) B (eV) | Energy of Peak C (eV) |
|---|---|---|---|
| [5,6]C60O | 540.81 | - | 542.11 |
| [6,6]C60O | 540.53 | 541.14 | 541.75 |
| C60H(OH) | 540.11 | 540.54 | 541.33 |
| C60-O-C60 | 539.87 | 540.89 | 541.10 |
| C60H-O-C60H | 539.53 | 539.94 | 540.76 |
| C59N(OH) | 540.29 | - | 541.44 |
| C59N-O-C59N | 539.81 | - | 541.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, S.; Guo, J.; Wu, Z.; Guo, C.; Cai, S.; Deng, M. Core-Hole Excitation Spectra of the Oxides and Hydrates of Fullerene C60 and Azafullerene C59N. Molecules 2024, 29, 609. https://doi.org/10.3390/molecules29030609
Li X, Wang S, Guo J, Wu Z, Guo C, Cai S, Deng M. Core-Hole Excitation Spectra of the Oxides and Hydrates of Fullerene C60 and Azafullerene C59N. Molecules. 2024; 29(3):609. https://doi.org/10.3390/molecules29030609
Chicago/Turabian StyleLi, Xiong, Shuyi Wang, Jingdong Guo, Ziye Wu, Changrui Guo, Shaohong Cai, and Mingsen Deng. 2024. "Core-Hole Excitation Spectra of the Oxides and Hydrates of Fullerene C60 and Azafullerene C59N" Molecules 29, no. 3: 609. https://doi.org/10.3390/molecules29030609
APA StyleLi, X., Wang, S., Guo, J., Wu, Z., Guo, C., Cai, S., & Deng, M. (2024). Core-Hole Excitation Spectra of the Oxides and Hydrates of Fullerene C60 and Azafullerene C59N. Molecules, 29(3), 609. https://doi.org/10.3390/molecules29030609