Chlorogenic Acid Attenuates Isoproterenol Hydrochloride-Induced Cardiac Hypertrophy in AC16 Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway
Abstract
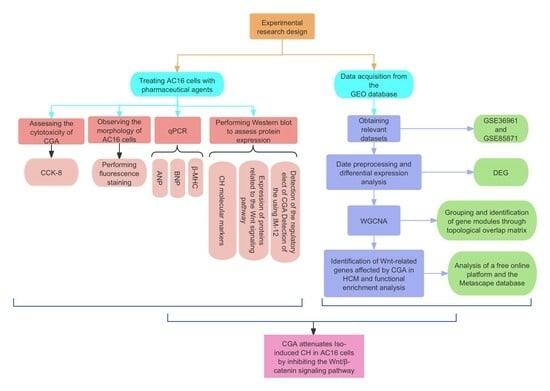
:1. Introduction
2. Results
2.1. Effects of CGA on the Viability of AC16 Cells
2.2. Effect of CGA on the Surface Area of AC16 Cells
2.3. Effect of CGA on the Transcription of ANP, BNP, and β-MHC in AC16 Cells
2.4. Effect of CGA on the Protein Expression Levels of ANP and BNP in AC16 Cells
2.5. Effect of CGA on the Wnt/β-Catenin Signaling Pathway
2.6. Effect of CGA and IM-12 on the Expression of Proteins in the Wnt Signaling Pathway
2.7. Identification of DEGs in HCM Tissue Compared to Healthy Tissue
2.8. WGCNA of the Whole Transcriptome Expression Matrix
2.9. Determination of Wnt-Related Genes Targeted by CGA in HCM-Affected Cells and Functional Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Kits
4.2. Culture of AC16 Cells
4.3. In Vitro CH Model and Drug Treatment
4.4. CCK-8 Assay
4.5. Cell Morphometric Analysis
4.6. Quantitative Reverse Transcription–Polymerase Chain Reaction (qPCR)
4.7. Western Blot Analysis
4.8. Data Acquisition from the GEO Database
4.9. Data Preprocessing and Differential Expression Analysis
4.10. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.11. Identification of Wnt-Related Genes Affected by CGA in HCM-Affected Cells and Functional Enrichment Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catapano, A.L.; Daccord, M.; Damato, E.; Humphries, S.E.; Neely, R.D.G.; Nordestgaard, B.G.; Pistollato, M.; Steinhagen-Thiessen, E. How should public health recommendations address Lp(a) measurement, a causative risk factor for cardiovascular disease (CVD)? Atherosclerosis 2022, 349, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, S.; Jeemon, P.; Mini, G.K.; Thankappan, K.R.; Sylaja, P.G.B.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Chien, K.R. Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 1999, 341, 1276–1283. [Google Scholar] [CrossRef]
- Barry, S.; Townsend, P. What causes a broken heart--molecular insights into heart failure. Int. Rev. Cell Mol. Biol. 2010, 284, 113–179. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liang, Q.; Chen, H.; Li, Y.; Huang, X.; Chen, Y. Research progress on the molecular mechanism of pathological cardiac hypertrophy. Lingnan J. Cardiovasc. Dis. 2021, 27, 753–756. [Google Scholar]
- Park, C.; Park, E.H.; Kang, J.; Zaheer, J.; Lee, H.G.; Lee, C.H.; Chang, K.; Hong, K.S. MR Assessment of Acute Pathologic Process after Myocardial Infarction in a Permanent Ligation Mouse Model: Role of Magnetic Nanoparticle-Contrasted MRI. Contrast Media Mol. Imaging 2017, 2017, 2870802. [Google Scholar] [CrossRef]
- Azakie, A.; Fineman, J.; He, Y. Myocardial transcription factors are modulated during pathologic cardiac hypertrophy in vivo. J. Thorac. Cardiovasc. Surg. 2006, 132, 1262–1271. [Google Scholar] [CrossRef]
- Liang, Q.; De Windt, L.; Witt, S.; Kimball, T.; Markham, B.; Molkentin, J. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J. Biol. Chem. 2001, 276, 30245–30253. [Google Scholar] [CrossRef]
- Hautala, N.; Tokola, H.; Luodonpää, M.; Puhakka, J.; Romppanen, H.; Vuolteenaho, O.; Ruskoaho, H. Pressure overload increases GATA4 binding activity via endothelin-1. Circulation 2001, 103, 730–735. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, M.; Zhang, Y. Role of GATA-4 in cardiac development and remodeling. Sheng Li Ke Xue Jin Zhan [Prog. Physiol.] 2008, 39, 302–306. [Google Scholar] [PubMed]
- Tang, C.; Liu, F.; Zhu, J.; Fu, Y.; Lin, Q.; Deng, C.; Hu, Z.; Yang, H.; Zheng, X.; Cheng, J.; et al. Myocyte-specific enhancer factor 2C: A novel target gene of miR-214-3p in suppressing angiotensin II-induced cardiomyocyte hypertrophy. Sci. Rep. 2016, 6, 36146. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.; Balza, R.; Xiao, Q.; Misra, R. SRF-dependent gene expression in isolated cardiomyocytes: Regulation of genes involved in cardiac hypertrophy. J. Mol. Cell. Cardiol. 2005, 39, 479–489. [Google Scholar] [CrossRef]
- Frey, N.; Olson, E. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef]
- Kang, Y. Cardiac hypertrophy: A risk factor for QT-prolongation and cardiac sudden death. Toxicol. Pathol. 2006, 34, 58–66. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Ma, Z.; Zhou, H.; Ni, J.; Liao, H.; Tang, Q. Genistein attenuates pathological cardiac hypertrophy in vivo and in vitro. Herz 2019, 44, 247–256. [Google Scholar] [CrossRef]
- Gessert, S.; Kühl, M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. 2010, 107, 186–199. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M. WNT signaling in adult cardiac hypertrophy and remodeling: Lessons learned from cardiac development. Circ. Res. 2010, 107, 1198–1208. [Google Scholar] [CrossRef]
- Dawson, K.; Aflaki, M.; Nattel, S. Role of the Wnt-Frizzled system in cardiac pathophysiology: A rapidly developing, poorly understood area with enormous potential. J. Physiol. 2013, 591, 1409–1432. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef]
- Ansley, D.; Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013, 229, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, Q.; Mou, Y.; Chen, X.; Yu, J. Research progress on the role of Wnt signaling pathway in cardiac hypertrophy. Med. Rev. 2021, 27, 1717–1721+1727. [Google Scholar]
- Malekar, P.; Hagenmueller, M.; Anyanwu, A.; Buss, S.; Streit, M.R.; Weiss, C.S.; Wolf, D.; Riffel, J.; Bauer, A.; Katus, H.A.; et al. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension 2010, 55, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Pahnke, A.; Conant, G.; Huyer, L.; Zhao, Y.; Feric, N.; Radisic, M. The role of Wnt regulation in heart development, cardiac repair and disease: A tissue engineering perspective. Biochem. Biophys. Res. Commun. 2016, 473, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef]
- Ni, B.; Sun, M.; Zhao, J.; Wang, J.; Cao, Z. The role of β-catenin in cardiac diseases. Front. Pharmacol. 2023, 14, 1157043. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Zhou, L.; Liu, Y. An essential role for Wnt/β-catenin signaling in mediating hypertensive heart disease. Sci. Rep. 2018, 8, 8996. [Google Scholar] [CrossRef]
- Hardt, S.; Sadoshima, J. Negative regulators of cardiac hypertrophy. Cardiovasc. Res. 2004, 63, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Hardt, S.; Sadoshima, J. Glycogen synthase kinase-3beta: A novel regulator of cardiac hypertrophy and development. Circ. Res. 2002, 90, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- van de Schans, V.; van den Borne, S.; Strzelecka, A.; Janssen, B.; van der Velden, J.; Langen, R.; Wynshaw-Boris, A.; Smits, J.; Blankesteijn, W. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension 2007, 49, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, X.; Guo, H.; Xu, P.; Li, F.; Fotina, T. Research progress on the accumulation and biosynthesis of chlorogenic acid in honeysuckle. Food Ind. 2021, 42, 252–256. [Google Scholar]
- Yu, P.; Li, D.; Xiao, W.; Zhao, L. Research progress on the structure-activity relationship of chlorogenic acid derivatives. Chin. J. Med. Chem. 2018, 28, 144–156+163. [Google Scholar] [CrossRef]
- Wang, Q.; Du, T.; Zhang, Z.; Ji, M.; Hu, H.; Chen, X. Research progress on the pharmacological effects and mechanisms of chlorogenic acid. Acta Pharm. Sin. 2020, 55, 2273–2280. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Zhai, Y.; Gao, Y.; Zhao, B. Research progress on chlorogenic acid pharmacokinetics. Chin. J. Tradit. Chin. Med. 2020, 35, 5095–5099. [Google Scholar]
- Wang, S.; Liu, Y. Exploration of the physical and chemical properties and biological activity of chlorogenic acid in honeysuckle. Rural. Pract. Technol. 2020, 6, 98–99. [Google Scholar]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Yanli, C.; Kai, H.; Fan, Y.; Yanyan, G.; Yan, Z.; Sen, L.; Bin, L.; Shuzhen, G. Metabolites of chlorogenic acid and its isomers: Metabolic pathways and activities for ameliorating myocardial hypertrophy. J. Funct. Foods 2022, 96, 105216. [Google Scholar]
- Zhang, J.; Xu, X.; Zhang, S.; Bao, X.; Zhang, L.; Yong, Z.; Li, Q. Use of Chlorogenic Acid in Preparing Drugs with Liver Protection Effect. Patent CN1899279, 24 January 2007. Patent Category Name: Invention Open, Applicant: Sichuan Jiuzhang Bio-Chemical Technology Development Co., Ltd. [Google Scholar]
- Chen, W.; Ju, W.; Tan, H. In vivo processes and drug interactions of chlorogenic acid. Pharmacol. Clin. Tradit. Chin. Med. 2008, 118–120. Available online: https://kns.cnki.net/kcms2/article/abstract?v=-0THPtffOh1Um2Qi3QtOEnunq5kGicRtu7kBfDWPU8f_nO5soUlQWHQesmijJN_sZ3O-fmo3ngbiu7L9_1D9jr1lDY6cqGCrgkUk4p7ud3N4g2wmSPcOSs791Com2R1jcqNnhCVk8C-csrxcUYX5vm19_iT69rgT&uniplatform=NZKPT (accessed on 8 November 2023).
- Xin-Pu, L.; Jie, Y.; Jin-Yin, L.; Hong-Sheng, L.; Fu-Jie, H.; Xing-Guo, C.; Zhi-De, H. Determination and pharmacokinetic study of chlorogenic acid in rat dosed with Yin-Huang granules by RP-HPLC. Biomed. Chromatogr. BMC 2006, 20, 206–210. [Google Scholar]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Chlorogenic acid inhibits the TLR4/NF-κB pathway and induces apoptosis of macrophage-derived foam cells. North. Pharm. 2019, 16, 123–126. [Google Scholar]
- Wang, B. Chlorogenic Acid Activates Autophagy to Inhibit Inflammation and Oxidative Stress by Inhibiting the TLR4/MAPK/NFκB Pathway. Master’s Thesis, Jilin University, Changchun, China, 2021. [Google Scholar]
- Gao, R. Anti-Mastitis Effect and Mechanism of Chlorogenic Acid. Ph.D. Dissertation, Jilin University, Changchun, China, 2014. [Google Scholar]
- Wang, T.; Duan, Y.; Fu, Q.; Liu, T.; Yu, J.; Sui, Z.; Huang, L.; Wen, G. IM-12 activates the Wnt-β-catenin signaling pathway and attenuates rtPA-induced hemorrhagic transformation in rats after acute ischemic stroke. Biochem. Cell Biol. = Biochim. Et Biol. Cell. 2019, 97, 702–708. [Google Scholar] [CrossRef]
- Mohamed, T.; Ang, Y.; Radzinsky, E.; Zhou, P.; Huang, Y.; Elfenbein, A.; Foley, A.; Magnitsky, S.; Srivastava, D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 2018, 173, 104–116.e12. [Google Scholar] [CrossRef]
- Foglia, M.; Poss, K. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef]
- Han, B.; Xu, J.; Shi, X.; Zheng, Z.; Shi, F.; Jiang, F.; Han, J. DL-3-n-Butylphthalide Attenuates Myocardial Hypertrophy by Targeting Gasdermin D and Inhibiting Gasdermin D Mediated Inflammation. Front. Pharmacol. 2021, 12, 688140. [Google Scholar] [CrossRef]
- Shah, A.; Bhullar, S.; Elimban, V.; Dhalla, N. Oxidative Stress as A Mechanism for Functional Alterations in Cardiac Hypertrophy and Heart Failure. Antioxidants 2021, 10, 931. [Google Scholar] [CrossRef]
- Bovolenta, P.; Esteve, P.; Ruiz, J.; Cisneros, E.; Lopez-Rios, J. Beyond Wnt inhibition: New functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 2008, 121, 737–746. [Google Scholar] [CrossRef]
- ter Horst, P.; Smits, J.; Blankesteijn, W. The Wnt/Frizzled pathway as a therapeutic target for cardiac hypertrophy: Where do we stand? Acta Physiol. 2012, 204, 110–117. [Google Scholar] [CrossRef]
- Fan, J.; Qiu, L.; Shu, H.; Ma, B.; Hagenmueller, M.; Riffel, J.; Meryer, S.; Zhang, M.; Hardt, S.; Wang, L.; et al. Recombinant frizzled1 protein attenuated cardiac hypertrophy after myocardial infarction via the canonical Wnt signaling pathway. Oncotarget 2018, 9, 3069–3080. [Google Scholar] [CrossRef]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic Acid Enhances Doxorubicin-Mediated Cytotoxic Effect in Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Hou, F.; Zhou, L.; Liu, Y. Wnt/β-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. 2019, 95, 815–829. [Google Scholar] [CrossRef]
- Villota, H.; Santa-González, G.A.; Uribe, D.; Henao, I.C.; Arroyave-Ospina, J.C.; Barrera-Causil, C.J.; Pedroza-Díaz, J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients 2022, 14, 4880. [Google Scholar] [CrossRef]
- Xue, W.; Hao, J.; Zhang, Q.; Jin, R.; Luo, Z.; Yang, X.; Liu, Y.; Lu, Q.; Ouyang, Y.; Guo, H. Chlorogenic Acid Inhibits Epithelial-Mesenchymal Transition and Invasion of Breast Cancer by Down-Regulating LRP6. J. Pharmacol. Exp. Ther. 2023, 384, 254–264. [Google Scholar] [CrossRef]
- Benjamin, I.J.; Jalil, J.E.; Tan, L.; Cho, K.; Weber, K.T.; Clark, W.A. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ. Res. 1989, 65, 657–670. [Google Scholar] [CrossRef]
- Sowah, D.; Brown, B.F.; Quon, A.; Alvarez, B.V.; Casey, J.R. Resistance to cardiomyocyte hypertrophy in ae3-/- mice, deficient in the AE3 Cl-/HCO3- exchanger. BMC Cardiovasc. Disord. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Ge, Z.; Lin, J.; Liu, J.; Yuan, X.; Lin, Z. GDF11 Attenuated ANG II-Induced Hypertrophic Cardiomyopathy and Expression of ANP, BNP and Beta-MHC Through Down- Regulating CCL11 in Mice. Curr. Mol. Med. 2018, 18, 661–671. [Google Scholar] [CrossRef]
- Morita, H.; Komuro, I. Heart Failure as an Aging-Related Phenotype. Int. Heart J. 2018, 59, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rochais, F.; Mesbah, K.; Kelly, R. Signaling pathways controlling second heart field development. Circ. Res. 2009, 104, 933–942. [Google Scholar] [CrossRef]
- Kohn, A.; Moon, R. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium 2005, 38, 439–446. [Google Scholar] [CrossRef]
- Acebron, S.; Niehrs, C. β-Catenin-Independent Roles of Wnt/LRP6 Signaling. Trends Cell Biol. 2016, 26, 956–967. [Google Scholar] [CrossRef]
- Kanemoto, S.; Matsubara, M.; Noma, M.; Leshnower, B.; Parish, L.; Jackson, B.; Hinmon, R.; Hamamoto, H.; Gorman, J.; Gorman, R. Mild hypothermia to limit myocardial ischemia-reperfusion injury: Importance of timing. Ann. Thorac. Surg. 2009, 87, 157–163. [Google Scholar] [CrossRef]
- Chen, Y.; Whetstone, H.; Youn, A.; Nadesan, P.; Chow, E.; Lin, A.; Alman, B. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J. Biol. Chem. 2007, 282, 526–533. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, L.; Thrasher, J.; Du, J.; Li, B. Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol. Cancer Ther. 2003, 2, 1215–1222. [Google Scholar]
- Seidensticker, M.; Behrens, J. Biochemical interactions in the wnt pathway. Biochim. Et Biophys. Acta 2000, 1495, 168–182. [Google Scholar] [CrossRef]
- Guan, X.; He, Y.; Wei, Z.; Shi, C.; Li, Y.; Zhao, R.; Pan, L.; Han, Y.; Hou, T.; Yang, J. Crosstalk between Wnt/β-catenin signaling and NF-κB signaling contributes to apical periodontitis. Int. Immunopharmacol. 2021, 98, 107843. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Yang, W.; Li, L.; Liu, H.; Tan, Y.; Ooi, S.; Chi, L.; Filion, L.; Figeys, D.; Wang, L. β-Catenin and NF-κB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ. 2015, 22, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Ye, B.; Li, X.; Margulies, K.B.; Xu, H.; Wang, X.; Li, F. Transcription Factor 7-like 2 Mediates Canonical Wnt/β-Catenin Signaling and c-Myc Upregulation in Heart Failure. Circ. Heart Fail 2016, 9, e003010. [Google Scholar] [CrossRef]
- Seferović, P.; Petrie, M.; Filippatos, G.; Anker, S.; Rosano, G.; Bauersachs, J.; Paulus, W.; Komajda, M.; Cosentino, F.; de Boer, R.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Tao, J.; Chen, W. RNF6 promotes colorectal cancer invasion and migration via the Wnt/β-catenin pathway by inhibiting GSK3β activity. Pathol. Res. Pract. 2021, 225, 153545. [Google Scholar] [CrossRef]
- Schmöle, A.; Brennführer, A.; Karapetyan, G.; Jaster, R.; Pews-Davtyan, A.; Hübner, R.; Ortinau, S.; Beller, M.; Rolfs, A.; Frech, M. Novel indolylmaleimide acts as GSK-3beta inhibitor in human neural progenitor cells. Bioorganic Med. Chem. 2010, 18, 6785–6795. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, G.; Lee, T.; Kim, M.; Kim, J.; Kwon, H.; Kim, J.; Jeong, W.; Lee, U.; Na, C.; et al. PARsylated transcription factor EB (TFEB) regulates the expression of a subset of Wnt target genes by forming a complex with β-catenin-TCF/LEF1. Cell Death Differ. 2021, 28, 2555–2570. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev. Res. 2019, 80, 294–309. [Google Scholar] [CrossRef]
- Davidson, M.; Nesti, C.; Palenzuela, L.; Walker, W.; Hernandez, E.; Protas, L.; Hirano, M.; Isaac, N. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 39, 133–147. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, Z.; Wu, Q.; Jiang, X.; Yuan, Y.; Chang, W.; Bian, Z.; Zhu, J.; Tang, Q. Cucurbitacin B Protects Against Pressure Overload Induced Cardiac Hypertrophy. J. Cell. Biochem. 2017, 118, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]









| Primer Name | Primer Sequence |
|---|---|
| ANP | Forward primer, 5′-CAGCAAGCAGTGGATTGCTCCT-3′ Reverse primer, 5′-TCTGCGTTGGACACGGCATTGT-3′ |
| BNP | Forward primer, 5′-TGGAAACGTCCGGGTTACAGGA-3′ Reverse primer, 5′-TCCGGTCCATCTTCCTCCCAAA-3′ |
| β-MHC | Forward primer, 5′-GGGCAAAGGCAAGGCCAAGAAA-3′ Reverse primer, 5′-ATGGGTGGAGCGCAAGTTGGTCA-3′ |
| GAPDH | Forward primer, 5′-GGAGCGAGATCCCTCCAAAAT-3′ Reverse primer, 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Wang, X.; Li, T.; Li, Y.; Ma, L. Chlorogenic Acid Attenuates Isoproterenol Hydrochloride-Induced Cardiac Hypertrophy in AC16 Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Molecules 2024, 29, 760. https://doi.org/10.3390/molecules29040760
He K, Wang X, Li T, Li Y, Ma L. Chlorogenic Acid Attenuates Isoproterenol Hydrochloride-Induced Cardiac Hypertrophy in AC16 Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Molecules. 2024; 29(4):760. https://doi.org/10.3390/molecules29040760
Chicago/Turabian StyleHe, Kai, Xiaoying Wang, Tingting Li, Yanfei Li, and Linlin Ma. 2024. "Chlorogenic Acid Attenuates Isoproterenol Hydrochloride-Induced Cardiac Hypertrophy in AC16 Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway" Molecules 29, no. 4: 760. https://doi.org/10.3390/molecules29040760
APA StyleHe, K., Wang, X., Li, T., Li, Y., & Ma, L. (2024). Chlorogenic Acid Attenuates Isoproterenol Hydrochloride-Induced Cardiac Hypertrophy in AC16 Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Molecules, 29(4), 760. https://doi.org/10.3390/molecules29040760