Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice
Abstract
:1. Introduction
2. Results
2.1. Effect of OXY on the Production of NO and Polarization in BV-2 Cells
2.2. OXY Improved Behavioral Impairments in LPS-Induced Mice
2.3. OXY Alleviated LPS-Induced Nerve Cells Injury
2.4. OXY Alleviated LPS-Induced Oxidative Stress in Brain
2.5. OXY Alleviated LPS-Induced Neuroinflammation in Brain
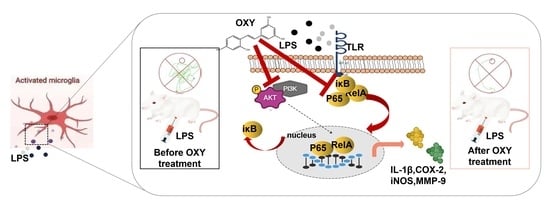
2.6. OXY Inhibited the Activation of the PI3K-Akt Signaling Pathway (Network Pharmacology Analysis)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cell Viability and Measurement of NO Production
3.4. Immunofluorescence Staining
3.5. Animal Study
3.6. Histopathology and Immunohistochemistry
3.7. Enzyme-Linked Immunosorbent Assay (ELISA)
3.8. Real-Time PCR Analysis
3.9. Network Pharmacology
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | amyloid β |
| AD | Alzheimer disease |
| BBB | Blood Brain Barrier |
| CNS | central nervous system |
| COX-2 | cyclooxygenase-2 |
| IL-1β | interleukin 1 beta |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharide |
| MMP9 | matrix metalloproteinase 9 |
| OXY | oxyresveratrol |
| PVDF | polyvinylidene fluoride |
| Res | resveratrol |
| ROS | reactive oxygen species |
| SDS | sodium dodecyl sulfate |
| SOD | superoxide dismutase |
| TNF-α | tumor necrosis factor alpha |
References
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Fu, W.-Y.; Wang, X.; Ip, N.Y. Targeting Neuroinflammation as a Therapeutic Strategy for Alzheimer’s Disease: Mechanisms, Drug Candidates, and New Opportunities. ACS Chem. Neurosci. 2019, 10, 872–879. [Google Scholar] [CrossRef]
- Luo, X.G.; Chen, S.D. The changing phenotype of microglia from homeostasis to disease. Transl. Neurodegener. 2012, 1, 9. [Google Scholar] [CrossRef]
- Skaper, S.D.; Laura, F.; Morena, Z.; Pietro, G. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Hagemeyer, N.; Prinz, M. The force awakens: Insights into the origin and formation of microglia. Curr. Opin. Neurobiol. 2016, 39, 30–37. [Google Scholar] [CrossRef]
- Salem, M.A.; Budzynska, B.; Kowalczyk, J.; El Sayed, N.S.; Mansour, S.M. Tadalafil and bergapten mitigate streptozotocin-induced sporadic Alzheimer’s disease in mice via modulating neuroinflammation, PI3K/Akt, Wnt/beta-catenin, AMPK/mTOR signaling pathways. Toxicol. Appl. Pharmacol. 2021, 429, 115697. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, Y.-Y.; Huang, S.; Xiao, L.-H.; Zhou, W.; Zhang, L.-Y.; Li, C.; Zhou, R.-P.; Tang, J.; Du, Z.-Y.; et al. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. Mol. Nutr. Food Res. 2018, 62, 1700281. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, M.; Hu, J.; Du, Z.; Su, Q.; Xiang, Z. Luteolin Suppresses Microglia Neuroinflammatory Responses and Relieves Inflammation-Induced Cognitive Impairments. Neurotox Res. 2021, 39, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhou, W.; Yue, H.; Zhang, C.; Ou, Y.T.; Yang, Z.J.; Hu, W.H. Compound AD110 Acts as Therapeutic Management for Alzheimer’s Disease and Stroke in Mouse and Rat Models. ACS Chem. Neurosci. 2020, 11, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhong, G.; Fu, S.; Xie, H.; Chi, T.; Li, L.; Rao, X.; Zeng, S.; Xu, D.; Wang, H.; et al. Microglia-Based Phenotypic Screening Identifies a Novel Inhibitor of Neuroinflammation Effective in Alzheimer’s Disease Models. ACS Chem. Neurosci. 2016, 7, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.G.A.A.; Lin, H.S. Oxyresveratrol: A bioavailable dietary polyphenol. J. Funct. Foods 2016, 22, 122–131. [Google Scholar] [CrossRef]
- Deng, H.; He, X.; Xu, Y.; Hu, X. Oxyresveratrol from Mulberry as a dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1318–o1319. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S. Piperine Derived from Piper nigrum L. Inhibits LPS-Induced Inflammatory through the MAPK and NF-κB Signalling Pathways in RAW264.7. Cells 2022, 11, 2990. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Bae, Y.S. The SYK side of TLR4: Signalling mechanisms in response to LPS and minimally oxidized LDL. Br. J. Pharmacol. 2012, 167, 990–999. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Ramis, M.R.; Tejada, S.; Jimenez-García, M.; Esteban, S.; Miralles, A.; Moranta, D. Resveratrol improves episodic-like memory and motor coordination through modulating neuroinflammation in old rats. J. Funct. Foods 2023, 104, 105533. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Ma, S.; Xie, Y.; Liu, H.; Yao, M.; Zhang, Y.; Yang, G.L.; Yang, B.; Guo, R.; et al. Resveratrol Preincubation Enhances the Therapeutic Efficacy of hUC-MSCs by Improving Cell Migration and Modulating Neuroinflammation Mediated by MAPK Signaling in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2020, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L. Functional brain imaging of episodic memory decline in ageing. J. Intern. Med. 2017, 281, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, L.J.; Huang, T.Q.; Kim, J.; Gu, M.Y.; Yang, H.O. Narciclasine inhibits LPS-induced neuroinflammation by modulating the Akt/IKK/NF-kappaB and JNK signaling pathways. Phytomedicine 2021, 85, 153540. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Zhang, X.; Zhang, X.; Qian, Y.; Ding, H.; Zhang, S. Stabilization of Brain Mast Cells Alleviates LPS-Induced Neuroinflammation by Inhibiting Microglia Activation. Front. Cell. Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.J.P.; Kinoshita, P.F.; e Silva, L.N.D.; Busch, M.D.S.; Atella, G.C.; Scavone, C.; Cortes, V.F.; Barbosa, L.A.; Santos, H.D.L. Ouabain attenuates oxidative stress and modulates lipid composition in hippocampus of rats in lipopolysaccharide-induced hypocampal neuroinflammation in rats. J. Cell. Biochem. 2019, 120, 4081–4091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, G.; Bai, Y.; Tao, Y.; Wang, L.; Yang, L.; Wu, H.; Huang, F.; Shi, H.; Wu, X. Natural bear bile powder suppresses neuroinflammation in lipopolysaccharide-treated mice via regulating TGR5/AKT/NF-kappaB signaling pathway. J. Ethnopharmacol. 2022, 289, 115063. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, N.; Xu, S.; Luo, Y.; Li, Y.; Jin, H.; Yu, C.; Shi, J.; Jin, F. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J. Nutr. Biochem. 2021, 88, 108552. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Dong, Q.-X.; Zhu, J.; Sun, X.; Zhang, L.-F.; Qiu, M.; Yu, X.-L.; Liu, R.-T. Resveratrol Rescues Tau-Induced Cognitive Deficits and Neuropathology in a Mouse Model of Tauopathy. Curr. Alzheimer Res. 2019, 16, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Yu, X.L.; Ji, M.; Liu, S.Y.; Wu, X.L.; Wang, Y.J.; Liu, R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T alpha-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef] [PubMed]
- Hankittichai, P.; Lou, H.J.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Oxyresveratrol Inhibits IL-1beta-Induced Inflammation via Suppressing AKT and ERK1/2 Activation in Human Microglia, HMC3. Int. J. Mol. Sci. 2020, 21, 6054. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef] [PubMed]








| Gene | Sequence (5′-3′) | |
|---|---|---|
| COX-2 | Forward: | ATAGACGAAATCAACAACCCCG |
| Reverse: | GGATTGGAAGTTCTATTGGCAG | |
| iNOS | Forward: | AGCTCGGGTTGAAGTGGTATG |
| Reverse: | CACAGCCACATTGATCTCCG | |
| MMP9 | Forward: | GCTGGCAGAGGCATACTTGTAC |
| Reverse: | GGTGTTCGAATGGCCTTTAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.; Pan, C.; Liu, H.; Dong, C.; Chang, X.; Zhou, W.; Wang, S.; Du, Z. Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice. Molecules 2024, 29, 1272. https://doi.org/10.3390/molecules29061272
Yin G, Pan C, Liu H, Dong C, Chang X, Zhou W, Wang S, Du Z. Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice. Molecules. 2024; 29(6):1272. https://doi.org/10.3390/molecules29061272
Chicago/Turabian StyleYin, Guangling, Chunxing Pan, Hong Liu, Changzhi Dong, Xia Chang, Wei Zhou, Shanshan Wang, and Zhiyun Du. 2024. "Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice" Molecules 29, no. 6: 1272. https://doi.org/10.3390/molecules29061272
APA StyleYin, G., Pan, C., Liu, H., Dong, C., Chang, X., Zhou, W., Wang, S., & Du, Z. (2024). Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice. Molecules, 29(6), 1272. https://doi.org/10.3390/molecules29061272