Preparation and Characterization of Pullulan-Based Packaging Paper for Fruit Preservation
Abstract
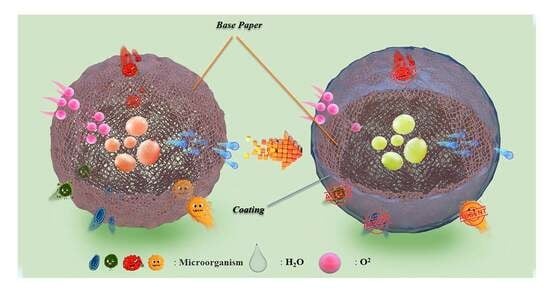
:1. Introduction
2. Results and Discussion
2.1. Micromorphology of the Packaging Papers
2.2. Oxygen- and Water Vapor-Barrier Properties of Different Types of Paper Samples
2.3. Free Radical-Scavenging Abilities and Antibacterial Properties of Different Types of Paper Samples
2.4. Mechanical Properties of Different Types of Paper Samples
2.5. Fruit Preservation Performance of Different Types of Paper Samples
2.5.1. Changes in the Weight Loss Rate and Hardness of Grapes
2.5.2. Changes in TSS and Soluble Protein Contents of Grapes
2.5.3. Changes in TA and Ascorbic Acid (AA) Contents of Grapes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pullulan-Based Packaging Papers
3.3. Scanning Electron Microscopy
3.4. Mechanical Stability Testing
3.5. Determination of WVTR
3.6. Determination of OTR
3.7. Antimicrobial Properties of the Packaging Papers
3.8. Antioxidant Property of the Packaging Papers
3.9. Fruit Property Testing
3.9.1. Weight Loss and Hardness of Fruits
3.9.2. TSS Analysis
3.9.3. TA Analysis
3.9.4. Soluble Protein
3.9.5. AA Analysis
3.10. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional bio-nanocomposite coatings for perishable fruits. Adv. Mater. 2020, 32, e1908291. [Google Scholar] [CrossRef]
- Kishore, K.; Pathak, K.A.; Shukla, R.; Bharali, R. Effect of storage temperature on physico-chemical and sensory attributes of purple passion fruit (Passiflora edulis Sims). J. Food Sci. Technol. 2011, 48, 484–488. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. Engl. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, N.; Chen, J.; Peng, X.; Wan, C. Chitosan-based coating enriched with hairy fig (Ficus hirta Vahl.) fruit extract for ‘Newhall’ navel orange preservation. Coatings 2018, 8, 445. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of le Conte pear. RSC Adv. 2021, 11, 9572–9585. [Google Scholar] [CrossRef]
- Cheng, H.; Mou, Z.; Wang, W.; Zhang, W.; Wang, Z.; Zhang, M.; Yang, E.; Sun, D. Chitosan–catechin coating as an antifungal and preservable agent for postharvest satsuma oranges. J. Food Biochem. 2019, 43, e12779. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Huang, P.; Li, H.; Liu, Y.; Zhang, Y.; Hu, X.; Deng, S.; Liu, Y.; Qin, W. Use of heat-shock and edible coating to improve the postharvest preservation of blueberries. Foods 2023, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, T.; Wei, N.; Pan, Z.; Wang, L. Incorporation of bayberry tannin into a locust bean gum/carboxycellulose nanocrystals/ZnO coating: Properties and its application in banana preservation. Polymers 2023, 15, 3364. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, J.; Zhang, F.; Zhang, R.; Zhang, X.; Zhong, K.; Yan, B. Development of mussel-inspired chitosan-derived edible coating for fruit preservation. Carbohydr. Polym. 2023, 321, 121293. [Google Scholar] [CrossRef]
- Shah, N.N.; Vishwasrao, C.; Singhal, R.S.; Ananthanarayan, L. n-Octenyl succinylation of pullulan: Effect on its physico-mechanical and thermal properties and application as an edible coating on fruits. Food Hydrocoll. 2016, 55, 179–188. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of sweet potato starch-based nanocomposite films activated with thyme essential oil on the shelf-life of baby spinach leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal−organic framework for antibacterial food packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liang, Q.; Rashid, A.; Qayum, A.; Chi, Z.; Ren, X.; Ma, H. Ultrasound-assisted development and characterization of novel polyphenol-loaded pullulan/trehalose composite films for fruit preservation. Ultrason. Sonochem. 2023, 92, 106242. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Zeng, W.C. Potato starch-based film incorporated with tea polyphenols and its application in fruit packaging. Polymers 2023, 15, 588. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Sarak, S.; Boonsuk, P.; Kantachote, D.; Kaewtatip, K. Film coating based on native starch and cationic starch blend improved postharvest quality of mangoes. Int. J. Biol. Macromol. 2022, 209, 125–131. [Google Scholar] [CrossRef]
- Kou, X.; Li, Y.; Wu, J.; Chen, Q.; Xue, Z. Effects of edible coatings on quality and antioxidant activity of Zizyphus jujuba Miller cv. Dongzao during storage. Trans. Tianjin Univ. 2017, 23, 51–61. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Tian, Y.; Li, L.; Wang, R.; Ji, N.; Ma, C.; Lei, J.; Guan, W.; Zhang, X. Pullulan-based active coating incorporating potassium metabisulfite maintains postharvest quality and induces disease resistance to soft rot in kiwifruit. Foods 2023, 12, 3197. [Google Scholar] [CrossRef]
- Dai, Y.H.; Wei, J.R.; Chen, X.Q. Interactions between tea polyphenols and nutrients in food. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3130–3150. [Google Scholar] [CrossRef]
- Pereira, J.F.; Lonni, A.A.S.G.; Mali, S. Development of biopolymeric films with addition of vitamin C and catuaba extract as natural antioxidants. Prep. Biochem. Biotechnol. 2022, 52, 1–10. [Google Scholar] [CrossRef]
- Ye, M.; Wang, S.; Ji, X.; Tian, Z.; Dai, L.; Si, C. Nanofibrillated cellulose-based superhydrophobic coating with antimicrobial performance. Adv. Compos. Hybrid Mater. 2023, 6, 30. [Google Scholar] [CrossRef]
- Xu, F.; Yun, D.; Huang, X.; Sun, B.; Tang, C.; Liu, J. Preparation, characterization, and application of pH-response color-changeable films based on pullulan, cooked amaranth (Amaranthus tricolor L.) juice, and bergamot essential oil. Foods 2023, 12, 2779. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chi, C. Development of pullulan/carboxylated cellulose nanocrystal/tea polyphenol bionanocomposite films for active food packaging. Int. J. Biol. Macromol. 2021, 186, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zeng, J.; Cheng, Z.; Wang, X.; Wang, B.; Zeng, Z.; Chen, K. Cellulose nanofibrils (CNFs) produced by different mechanical methods to improve mechanical properties of recycled paper. Carbohydr. Polym. 2021, 254, 117474. [Google Scholar] [CrossRef]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef]
- Kavoosi, G.; Derakhshan, M.; Salehi, M.; Rahmati, L. Microencapsulation of zataria essential oil in agar, alginate and carrageenan. Innov. Food Sci. Emerg. Technol. 2018, 45, 418–425. [Google Scholar] [CrossRef]
- Guo, W.; Li, W.; Yang, B.; Zhu, Z.; Liu, D.; Zhu, X. A novel noninvasive and cost-effective handheld detector on soluble solids content of fruits. J. Food Eng. 2019, 257, 1–9. [Google Scholar] [CrossRef]
- Maurizzi, E.; Bigi, F.; Volpelli, L.A.; Pulvirenti, A. Improving the post-harvest quality of fruits during storage through edible packaging based on guar gum and hydroxypropyl methylcellulose. Food Packag. Shelf Life 2023, 40, 101178. [Google Scholar] [CrossRef]
- Zheng, Y.; Duan, L.; Li, J.; Zhang, P.; Jiang, Y.; Yang, X.; Li, X.; Jia, X. Photocatalytic titanium dioxide reduces postharvest decay of nectarine fruit packaged in different materials through modulating central carbon and energy metabolisms. Food Chem. 2024, 433, 137357. [Google Scholar] [CrossRef]
- Chang, C.-K.; Lin, F.-Y.; Tsai, S.-Y.; Gavahian, M.; Cheng, K.-C.; Hou, C.-Y.; Chen, M.-H.; Santoso, S.P.; Hsieh, C.-W. Active packaging incorporating chitosan, casein phosphopeptide, and plasmonic modification to maintain quality and regulate the respiration rate of fresh-cut Pyrus serotina Rehder during storage. Food Packag. Shelf Life 2023, 40, 101223. [Google Scholar] [CrossRef]
- Nur, M.A.; Uddin, M.R.; Uddin, M.J.; Satter, M.A.; Amin, M.Z. Physiochemical and nutritional analysis of the two species of dragon fruits (Hylocereus sp.) cultivated in Bangladesh. S. Afr. J. Bot. 2023, 155, 103–109. [Google Scholar] [CrossRef]
- Neto, J.F.S.; Khan, S.; Filho, C.A.; Neto, A.G.; Torres, A.G.; Azevedo, E.P.P. Photostability of vitamin C in industrialized fruit juices and isomers determination by HPLC-DAD. J. Chromatogr. Open 2023, 4, 100103. [Google Scholar] [CrossRef]
- Moreton, N.; Puzio, M.; McCormack, J.; O’Connor, J.J. The effects of prolyl hydroxylase inhibition during and post, hypoxia, oxygen glucose deprivation and oxidative stress, in isolated rat hippocampal slices. Brain Res. Bull. 2023, 205, 110822. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Tian, Z.; Wang, S.; Ji, X.; Wang, D.; Ci, X. Simple preparation of environmentally friendly and durable superhydrophobic antibacterial paper. Cellulose 2023, 30, 2427–2440. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Li, L.; Jiang, K.; Gao, J.; Zhong, K.; Pan, M.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Goldberg, T.; Agra, H.E.; Ben-Arie, R. Non-destructive measurement of fruit firmness to predict the shelf-life of ‘Hayward’ kiwifruit. Sci. Horticult. 2019, 244, 339–342. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Tian, Z. Preparation and Characterization of Pullulan-Based Packaging Paper for Fruit Preservation. Molecules 2024, 29, 1394. https://doi.org/10.3390/molecules29061394
Dong H, Tian Z. Preparation and Characterization of Pullulan-Based Packaging Paper for Fruit Preservation. Molecules. 2024; 29(6):1394. https://doi.org/10.3390/molecules29061394
Chicago/Turabian StyleDong, Hang, and Zhongjian Tian. 2024. "Preparation and Characterization of Pullulan-Based Packaging Paper for Fruit Preservation" Molecules 29, no. 6: 1394. https://doi.org/10.3390/molecules29061394
APA StyleDong, H., & Tian, Z. (2024). Preparation and Characterization of Pullulan-Based Packaging Paper for Fruit Preservation. Molecules, 29(6), 1394. https://doi.org/10.3390/molecules29061394