Amino Sugar-Enriched Fraction of Korean Red Ginseng Extract Induces the Priming Step of NLRP3 Inflammasome
Abstract
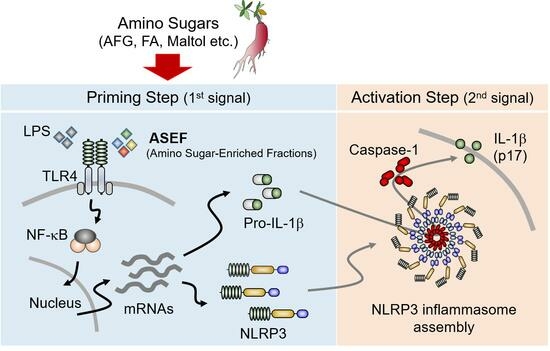
:1. Introduction
2. Results
2.1. ASEF Increases the Expression of Cytokines and NLRP3 Inflammasome Components
2.2. ASEF Induced the Levels of NLRP3, pro-IL-1β, and caspase-1
2.3. ASEF Stimulated the TLR4 Signaling Pathway, Resulting in the Upregulation of NLRP3 Inflammasome Components
2.4. Maltol, an Amino Sugar in RGE, Induced the Expression of pro-IL-1β and NLRP3 Inflammasome Components
2.5. ASEF Stimulated the Activation of the Inflammasome by Inducing the Priming Step of Inflammasome Activation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of ASEF, AFG, and FA
5.2. Analysis of Contents of Contents of ASEF
5.3. Cell Culturing
5.4. Cell Treatment
5.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time PCR (qPCR)
5.6. Western Blot Analysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Perrone, M.; Boncompagni, C.; Borghi, C.; Campagnaro, A.; Marchetti, F.; Anania, G.; Greco, P.; Fiorica, F.; Pinton, P.; et al. Targeting the NLRP3 Inflammasome as a New Therapeutic Option for Overcoming Cancer. Cancers 2021, 13, 2297. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Roles of ginsenosides in inflammasome activation. J. Ginseng Res. 2019, 43, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Lee, G.S. Korean Red Ginseng, a regulator of NLRP3 inflammasome, in the COVID-19 pandemic. J. Ginseng Res. 2022, 46, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Min, H. Ginseng, the ‘Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef]
- Scaglione, F.; Ferrara, F.; Dugnani, S.; Falchi, M.; Santoro, G.; Fraschini, F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Under Exp. Clin. Res. 1990, 16, 537–542. [Google Scholar]
- Han, B.C.; Ahn, H.; Lee, J.; Jeon, E.; Seo, S.; Jang, K.H.; Lee, S.H.; Kim, C.H.; Lee, G.S. Nonsaponin fractions of Korean Red Ginseng extracts prime activation of NLRP3 inflammasome. J. Ginseng Res. 2017, 41, 513–523. [Google Scholar] [CrossRef]
- Ahn, H.; Han, B.C.; Lee, S.H.; Lee, G.S. Fructose-arginine, a non-saponin molecule of Korean Red Ginseng, attenuates AIM2 inflammasome activation. J. Ginseng Res. 2020, 44, 808–814. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, G.; Han, B.C.; Lee, S.H.; Lee, G.S. Maltol, a Natural Flavor Enhancer, Inhibits NLRP3 and Non-Canonical Inflammasome Activation. Antioxidants 2022, 11, 1923. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Bartok, E.; Rieger, A.; Franchi, L.; Nunez, G.; Hornung, V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011, 187, 613–617. [Google Scholar] [CrossRef]
- McKee, C.M.; Coll, R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 2020, 108, 937–952. [Google Scholar] [CrossRef]
- Kim, H.G.; Yoo, S.R.; Park, H.J.; Lee, N.H.; Shin, J.W.; Sathyanath, R.; Cho, J.H.; Son, C.G. Antioxidant effects of Panax ginseng C.A. Meyer in healthy subjects: A randomized, placebo-controlled clinical trial. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Tang, Y.; Zhang, F.; Zhang, L. Roles of ginsenosides in sepsis. J. Ginseng Res. 2023, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, H.; Han, B.C.; Lee, S.H.; Cho, Y.W.; Kim, C.H.; Hong, E.J.; An, B.S.; Jeung, E.B.; Lee, G.S. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol. Lett. 2014, 158, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Cho, H.J.; Yi, Y.S. A novel mechanism of Korean Red Ginseng-mediated anti-inflammatory action via targeting caspase-11 non-canonical inflammasome in macrophages. J. Ginseng Res. 2022, 46, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, K.H.; Sohn, E.; Park, J.D.; Kim, B.O.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Bae, K.G.; Jung, I.S.; Kim, C.H.; Yun, Y.S.; Song, J.Y. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J. Infect. 2002, 45, 32–38. [Google Scholar] [CrossRef]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef]
- Ichinohe, T.; Yamazaki, T.; Koshiba, T.; Yanagi, Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. USA 2013, 110, 17963–17968. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Saba, E.; Lee, Y.Y.; Kim, M.; Kim, S.H.; Hong, S.B.; Rhee, M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J. Ginseng Res. 2018, 42, 577–584. [Google Scholar] [CrossRef]
- Ahn, H.; Han, B.C.; Kim, J.; Kang, S.G.; Kim, P.H.; Jang, K.H.; So, S.H.; Lee, S.H.; Lee, G.S. Nonsaponin fraction of Korean Red Ginseng attenuates cytokine production via inhibition of TLR4 expression. J. Ginseng Res. 2019, 43, 291–299. [Google Scholar] [CrossRef] [PubMed]
- In, G.; Ahn, N.G.; Bae, B.S.; Lee, M.W.; Park, H.W.; Jang, K.H.; Cho, B.G.; Han, C.K.; Park, C.K.; Kwak, Y.S. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J. Ginseng Res. 2017, 41, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.S.; Jo, S.H.; Kang, B.H.; Apostolidis, E.; Lee, M.S.; Jang, H.D.; Kwon, Y.I. In vitro and in vivo antihyperglycemic effect of 2 amadori rearrangement compounds, arginyl-fructose and arginyl-fructosyl-glucose. J. Food Sci. 2011, 76, H188–H193. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef]







| Arg | Amino Sugars | Ginsenosides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFG | FA | Maltol | Rg1 | Re | Rf | Rh1 | Rg2s | Rb1 | Rc | Rd | Rg3s | Rg3r | |
| 9.68 | 95.41 | 14.46 | 3.48 | 4.63 | 2.5 | 1.13 | 0.22 | 1.8 | 5.43 | 1.9 | 0.36 | 0.11 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.; Lee, G.-S. Amino Sugar-Enriched Fraction of Korean Red Ginseng Extract Induces the Priming Step of NLRP3 Inflammasome. Molecules 2024, 29, 1455. https://doi.org/10.3390/molecules29071455
Ahn H, Lee G-S. Amino Sugar-Enriched Fraction of Korean Red Ginseng Extract Induces the Priming Step of NLRP3 Inflammasome. Molecules. 2024; 29(7):1455. https://doi.org/10.3390/molecules29071455
Chicago/Turabian StyleAhn, Huijeong, and Geun-Shik Lee. 2024. "Amino Sugar-Enriched Fraction of Korean Red Ginseng Extract Induces the Priming Step of NLRP3 Inflammasome" Molecules 29, no. 7: 1455. https://doi.org/10.3390/molecules29071455
APA StyleAhn, H., & Lee, G. -S. (2024). Amino Sugar-Enriched Fraction of Korean Red Ginseng Extract Induces the Priming Step of NLRP3 Inflammasome. Molecules, 29(7), 1455. https://doi.org/10.3390/molecules29071455