Inflammatory-Targeted Lipid Carrier as a New Nanomaterial to Formulate an Inhaled Drug Delivery System
Abstract
:1. Introduction
2. Results and Discussion
2.1. PEA-LNPs Preparation and Characterization
2.2. In Vitro PEA Dissolution Rate
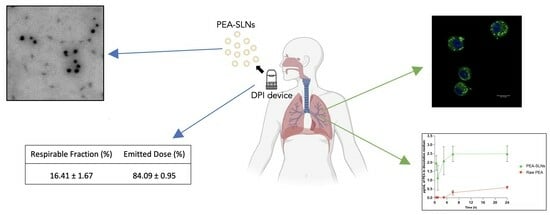
2.3. Freeze-Dry Powder Characterization and In Vitro Respirability
2.4. In Vitro Studies on Macrophage MH-S Cell Line
3. Materials and Methods
3.1. Materials
3.2. Lipid Nanoparticles Preparation
3.3. Morphology and Size
3.4. PEA Dissolution Rate
3.5. HPLC Analysis
3.6. Freeze-Dry Powder Characterization
3.6.1. Density
3.6.2. Flowability
3.6.3. In Vitro Respirability
3.7. Studies on Macrophage MH-S Cell Line
3.7.1. Cytotoxicity by MTT Test
3.7.2. Preparation of Coumarin-6 Labelled PEA-LNPs
3.7.3. Flow Cytometry
3.7.4. Confocal Microscopy
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghasafari, P.; George, U.; Pidaparti, R. A Review of Inflammatory Mechanism in Airway Diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sano, H.; Takita, K.; Morita, M.; Yamanaka, S.; Ichikawa, T.; Numakura, T.; Ida, T.; Jung, M.; Ogata, S.; et al. Supersulphides Provide Airway Protection in Viral and Chronic Lung Diseases. Nat. Commun. 2023, 14, 4476. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Nano- and Micro-Based Inhaled Drug Delivery Systems for Targeting Alveolar Macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Grimmett, M.E.; Domalewski, C.J.; Cui, H. Inhalable Nanotherapeutics to Improve Treatment Efficacy for Common Lung Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1586. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Gao, R.; Fang, Z.; Yang, W.; Tang, Z.; Wang, Q.; Wu, Y.; Fang, J.; Yu, W. Precise Nanodrug Delivery Systems with Cell-Specific Targeting for ALI/ARDS Treatment. Int. J. Pharm. 2023, 644, 123321. [Google Scholar] [CrossRef] [PubMed]
- Leo, E.; Maretti, E. Inhaled Lipid Nanoparticles: A Feasible Tool for a Challenging Route. Curr. Drug Deliv. 2024, 21, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.; Kaur, J.; De Rubis, G.; Paudel, K.R.; Prasher, P.; Patel, V.K.; Corrie, L.; Chellappan, D.K.; Gupta, G.; Singh, S.K.; et al. Inhalation Drug Delivery in Combating Pulmonary Infections: Advances and Challenges. J. Drug Deliv. Sci. Technol. 2023, 89, 105022. [Google Scholar] [CrossRef]
- ISMED Insmed Initiates Frontline Clinical Trial Program for ARIKAYCE® (Amikacin Liposome Inhalation Suspension) in Patients with MAC Lung Disease. Available online: https://investor.insmed.com/2021-01-04-Insmed-Initiates-Frontline-Clinical-Trial-Program-for-ARIKAYCE-R-amikacin-liposome-inhalation-suspension-in-Patients-with-MAC-Lung-Disease (accessed on 6 February 2024).
- FDA. FDA Approves a New Antibacterial Drug to Treat a Serious Lung Disease Using a Novel Pathway to Spur Innovation. Available online: https://www.amr-insights.eu/fda-approves-a-new-antibacterial-drug-to-treat-a-serious-lung-disease-using-a-novel-pathway-to-spur-innovation/ (accessed on 6 February 2024).
- Siafaka, P.I.; Özcan Bülbül, E.; Miliotou, A.N.; Karantas, I.D.; Okur, M.E.; Üstündağ Okur, N. Nano-Based Carriers for Pulmonary Drug Delivery: A Review on the Available Drug Delivery Applications and Toxicity Issues. J. Drug Deliv. Sci. Technol. 2024, 92, 105381. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, X.; Zhao, C.; Chen, R.; Chen, X.; Rao, L. Inhaled Drug Delivery: Past, Present, and Future. Nano Today 2023, 52, 101942. [Google Scholar] [CrossRef]
- Pitchai, A.; Buhman, K.; Shannahan, J.H. Lipid Mediators of Inhalation Exposure-Induced Pulmonary Toxicity and Inflammation. Inhal. Toxicol. 2024, 36, 57–74. [Google Scholar] [CrossRef]
- Molinari, S.; Maretti, E.; Battini, R.; Leo, E. Linking Endocannabinoid System, Palmitoylethanolamide and Sarcopenia in View of Therapeutic Outcomes. In Cannabis, Cannabinoids and Endocannabinoids; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef] [PubMed]
- García, L.F. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front. Immunol. 2020, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, L.; D’amico, R.; Cordaro, M.; Siracusa, R.; Fusco, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Coaccioli, S.; Genovese, T.; et al. Aerosol-Administered Adelmidrol Attenuates Lung Inflammation in a Murine Model of Acute Lung Injury. Biomolecules 2022, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Azhar Shekoufeh Bahari, L.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kłodzińska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-Mediated Pulmonary Drug Delivery: State of the Art towards Efficient Treatment of Recalcitrant Respiratory Tract Bacterial Infections. Drug Deliv. Transl. Res. 2021, 11, 1634–1654. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xie, Q.; Huang, X.; Ban, J.; Wang, B.; Wei, X.; Chen, Y.; Lu, Z. Increased Skin Permeation Efficiency of Imperatorin via Charged Ultradeformable Lipid Vesicles for Transdermal Delivery. Int. J. Nanomed. 2018, 13, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Maretti, E.; Molinari, S.; Battini, R.; Rustichelli, C.; Truzzi, E.; Iannuccelli, V.; Leo, E. Design, Characterization, and In Vitro Assays on Muscle Cells of Endocannabinoid-like Molecule Loaded Lipid Nanoparticles for a Therapeutic Anti-Inflammatory Approach to Sarcopenia. Pharmaceutics 2022, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Maretti, E.; Rustichelli, C.; Romagnoli, M.; Balducci, A.G.; Buttini, F.; Sacchetti, F.; Leo, E.; Iannuccelli, V. Solid Lipid Nanoparticle Assemblies (SLNas) for an Anti-TB Inhalation Treatment—A Design of Experiments Approach to Investigate the Influence of Pre-Freezing Conditions on the Powder Respirability. Int. J. Pharm. 2016, 511, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.-J.; Chen, X.D.; Pearce, D. Effect of Surface Composition on the Flowability of Industrial Spray-Dried Dairy Powders. Colloids Surf. B Biointerfaces 2005, 46, 182–187. [Google Scholar] [CrossRef]
- Edwards, D.A.; Hanes, J.; Caponetti, G.; Hrkach, J.; Ben-Jebria, A.; Eskew, M.L.; Mintzes, J.; Deaver, D.; Lotan, N.; Langer, R. Large Porous Particles for Pulmonary Drug Delivery. Science 1997, 276, 1868–1871. [Google Scholar] [CrossRef]
- Mohan, A.R.; Wang, Q.; Dhapare, S.; Bielski, E.; Kaviratna, A.; Han, L.; Boc, S.; Newman, B. Advancements in the Design and Development of Dry Powder Inhalers and Potential Implications for Generic Development. Pharmaceutics 2022, 14, 2495. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical Stability of Dry Powder Inhaler Formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Powder Flow 2.9.36. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2021.
- Sarraguça, M.C.; Cruz, A.V.; Soares, S.O.; Amaral, H.R.; Costa, P.C.; Lopes, J.A. Determination of Flow Properties of Pharmaceutical Powders by near Infrared Spectroscopy. J. Pharm. Biomed. Anal. 2010, 52, 484–492. [Google Scholar] [CrossRef]
- Kaleem, M.A.; Alam, M.Z.; Khan, M.; Jaffery, S.H.I.; Rashid, B. An Experimental Investigation on Accuracy of Hausner Ratio and Carr Index of Powders in Additive Manufacturing Processes. Met. Powder Rep. 2021, 76, S50–S54. [Google Scholar] [CrossRef]
- Amidon, G.E.; Meyer, P.J.; Mudie, D.M. Chapter 10—Particle, Powder, and Compact Characterization. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 271–293. ISBN 978-0-12-802447-8. [Google Scholar]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle van Der Waals Force in Powder Flowability and Compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- Thakral, S.; Sonje, J.; Munjal, B.; Bhatnagar, B.; Suryanarayanan, R. Mannitol as an Excipient for Lyophilized Injectable Formulations. J. Pharm. Sci. 2023, 112, 19–35. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Preparations for Inhalation (2.09.18). In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2021.
- Mehanna, M.M.; Mohyeldin, S.M.; Elgindy, N.A. Rifampicin-Carbohydrate Spray-Dried Nanocomposite: A Futuristic Multiparticulate Platform For Pulmonary Delivery. Int. J. Nanomed. 2019, 14, 9089–9112. [Google Scholar] [CrossRef] [PubMed]
- Syama, K.; Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Development of Lipid Nanoparticles and Liposomes Reference Materials (II): Cytotoxic Profiles. Sci. Rep. 2022, 12, 18071. [Google Scholar] [CrossRef] [PubMed]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Maretti, E.; Costantino, L.; Buttini, F.; Rustichelli, C.; Leo, E.; Truzzi, E.; Iannuccelli, V. Newly Synthesized Surfactants for Surface Mannosylation of Respirable SLN Assemblies to Target Macrophages in Tuberculosis Therapy. Drug Deliv. Transl. Res. 2019, 9, 298–310. [Google Scholar] [CrossRef]
- De Santana, N.S.; de Oliveira de Siqueira, L.B.; do Nascimento, T.; Santos-Oliveira, R.; dos Santos Matos, A.P.; Ricci-Júnior, E. Nanoparticles for the Treatment of Visceral Leishmaniasis: Review. J. Nanoparticle Res. 2023, 25, 24. [Google Scholar] [CrossRef]
- Chae, J.; Choi, Y.; Tanaka, M.; Choi, J. Inhalable Nanoparticles Delivery Targeting Alveolar Macrophages for the Treatment of Pulmonary Tuberculosis. J. Biosci. Bioeng. 2021, 132, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, E.; Nascimento, T.L.; Iannuccelli, V.; Costantino, L.; Lima, E.M.; Leo, E.; Siligardi, C.; Gualtieri, M.L.; Maretti, E. In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials 2020, 10, E568. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.; Pinheiro, M.; Drasler, B.; Septiadi, D.; Petri-Fink, A.; Santos, S.G.; Rothen-Rutishauser, B.; Reis, S. Lipid Nanoparticles Biocompatibility and Cellular Uptake in a 3D Human Lung Model. Nanomedicine 2020, 15, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, B.; Huang, Z.; Huang, Y.; Hu, P.; Pan, X.; Wu, C. Investigating the Effect of Particle Size on Cellular Uptake by Aggregation-Caused Quenching Probe–Encapsulating Solid Lipid Nanoparticles, Inhaled. J. Pharm. Innov. 2022, 17, 1109–1115. [Google Scholar] [CrossRef]
- Melamed, J.R.; Yerneni, S.S.; Arral, M.L.; LoPresti, S.T.; Chaudhary, N.; Sehrawat, A.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Weissman, D.; et al. Ionizable Lipid Nanoparticles Deliver mRNA to Pancreatic β Cells via Macrophage-Mediated Gene Transfer. Sci. Adv. 2023, 9, eade1444. [Google Scholar] [CrossRef]
- Pesce, M.; Seguella, L.; Cassarano, S.; Aurino, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Del Re, A.; Vincenzi, M.; Sarnelli, G.; et al. Phytotherapics in COVID19: Why Palmitoylethanolamide? Phytother. Res. 2020, 35, 2514–2522. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. Harnessing the Anti-Inflammatory Potential of Palmitoylethanolamide. Drug Discov. Today 2014, 19, 1632–1639. [Google Scholar] [CrossRef]
- Redlich, S.; Ribes, S.; Schütze, S.; Nau, R. Palmitoylethanolamide Stimulates Phagocytosis of Escherichia coli K1 by Macrophages and Increases the Resistance of Mice against Infections. J. Neuroinflamm. 2014, 11, 108. [Google Scholar] [CrossRef]
- Redlich, S.; Ribes, S.; Schütze, S.; Czesnik, D.; Nau, R. Palmitoylethanolamide Stimulates Phagocytosis of Escherichia Coli K1 and Streptococcus Pneumoniae R6 by Microglial Cells. J. Neuroimmunol. 2012, 244, 32–34. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- European Pharmacopoeia Bulk Density and Tapped Density of Powders (2.9.34). In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2021.




| Sample (Lipids/Mannitol Ratio) | Bulk Density (g/cm3) ± SD | Tap Density (g/cm3) ± SD | Housner Ratio | Carr’s Index (%) | Angle of Repose (°) ± SD |
|---|---|---|---|---|---|
|
C-LNPs (no mannitol) | 0.07 ± 0.02 | 0.08 ± 0.01 | 1.14 | 12.5 | 34.1 ± 1.7 |
|
PEA-LNPs (no mannitol) | 0.11 ± 0.01 | 0.16 ± 0.03 | 1.45 | 31 | 46.7 ± 2.7 |
|
PEA-LNPs (1:1 ratio) | 0.04 ± 0.01 | 0.05 ± 0.02 | 1.25 | 20 | 35.6 ± 4.2 |
|
PEA-LNPs (1:2 ratio) | 0.05 ± 0.01 | 0.05 ± 0.01 | 1.08 | 7 | 27.0 ± 4.8 |
| Sample (Lipids/Mannitol Ratio) | ED (%) ± SD | FPF (%) ± SD | LPF (%) ± SD |
|---|---|---|---|
|
PEA-LNPs (no mannitol) | 61.9 ± 3.9 | 0.1 ± 0.0 | 61.8 ± 2.8 |
|
PEA-LNPs (1:1 ratio) | 81.5 ± 2.5 | 9.6 ± 3.2 | 71.9 ± 2.3 |
|
PEA-LNPs (1:2 ratio) | 84.1 ± 1.0 | 16.4 ± 1.7 | 67.7 ± 0.8 |
| Sample | 1 h | 3 h |
|---|---|---|
| Fluorescence (%) | ||
| Untreated cells | 1.03 ± 0.09 | 0.49 ± 0.16 |
| Not labelled C-LNPs | 0.54 ± 0.18 | 0.43 ± 0.02 |
| Labelled PEA-LNPs | 94.65 ± 0.67 | 96.73 ± 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maretti, E.; Gioia, F.; Rustichelli, C.; Molinari, S.; Leo, E. Inflammatory-Targeted Lipid Carrier as a New Nanomaterial to Formulate an Inhaled Drug Delivery System. Molecules 2024, 29, 1616. https://doi.org/10.3390/molecules29071616
Maretti E, Gioia F, Rustichelli C, Molinari S, Leo E. Inflammatory-Targeted Lipid Carrier as a New Nanomaterial to Formulate an Inhaled Drug Delivery System. Molecules. 2024; 29(7):1616. https://doi.org/10.3390/molecules29071616
Chicago/Turabian StyleMaretti, Eleonora, Federica Gioia, Cecilia Rustichelli, Susanna Molinari, and Eliana Leo. 2024. "Inflammatory-Targeted Lipid Carrier as a New Nanomaterial to Formulate an Inhaled Drug Delivery System" Molecules 29, no. 7: 1616. https://doi.org/10.3390/molecules29071616
APA StyleMaretti, E., Gioia, F., Rustichelli, C., Molinari, S., & Leo, E. (2024). Inflammatory-Targeted Lipid Carrier as a New Nanomaterial to Formulate an Inhaled Drug Delivery System. Molecules, 29(7), 1616. https://doi.org/10.3390/molecules29071616