Green Synthesis of a Molecularly Imprinted Polymer Based on a Novel Thiophene-Derivative for Electrochemical Sensing
Abstract
:1. Introduction
2. Results and Discussion
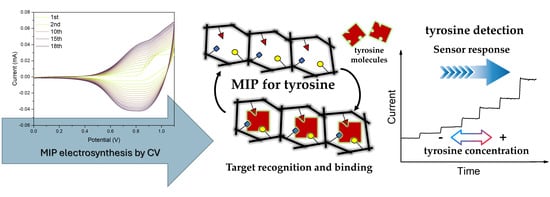
2.1. MIP Electrosynthesis
2.2. Optimization of Detection Parameters
2.3. Tyrosine Amperometric Detection
2.4. Sensor Selectivity, Stability and Real Sample Analyses
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Electrosynthesis of the MIP
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Mazzotta, E.; Di Giulio, T.; Malitesta, C. Electrochemical Sensing of Macromolecules Based on Molecularly Imprinted Polymers: Challenges, Successful Strategies, and Opportunities. Anal. Bioanal. Chem. 2022, 414, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, T.; Mazzotta, E.; Malitesta, C. Molecularly Imprinted Polyscopoletin for the Electrochemical Detection of the Chronic Disease Marker Lysozyme. Biosensors 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, T.; Barca, A.; Verri, T.; De Gennaro, M.; Giancane, G.; Mazzotta, E.; Malitesta, C. Molecular Imprinting Based on Metal-Ion Mediated Recognition: Electrosynthesis of Artificial Receptors for the Selective Detection of Peptides. Sens. Actuators B Chem. 2023, 133589. [Google Scholar] [CrossRef]
- Mazzotta, E.; Di Giulio, T.; Mariani, S.; Corsi, M.; Malitesta, C.; Barillaro, G. Vapor-Phase Synthesis of Molecularly Imprinted Polymers on Nanostructured Materials at Room-Temperature. Small 2023, 19, 2302274. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Row, K.H. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular Imprinting: Green Perspectives and Strategies. Adv. Mater. 2021, 33, 2100543. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, R.; Rebocho, S.; Casimiro, T. Green Strategies for Molecularly Imprinted Polymer Development. Polymers 2018, 10, 306. [Google Scholar] [CrossRef]
- Koteswararao, P.R.; Tulasi, S.L.; Pavani, Y. Impact of Solvents on Environmental Pollution. J. Chem. Pharm. Sci. 2014, 132–135. [Google Scholar]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [PubMed]
- Janík, R.; Kubov, M.; Schieber, B. The Ground-Level Ozone Concentration in Forest and Urban Environments in Central Slovakia. Environ. Monit. Assess. 2023, 195, 24. [Google Scholar] [CrossRef]
- Raj, A.; Chowdhury, A.; Ali, S.W. Green Chemistry: Its Opportunities and Challenges in Colouration and Chemical Finishing of Textiles. Sustain. Chem. Pharm. 2022, 27, 100689. [Google Scholar] [CrossRef]
- Svenson, J. Ultrasound-Assisted Preparation of Molecularly Imprinted Polymers: Effects on Polymer Morphology, Binding, and Chromatographic Behavior. Anal. Lett. 2006, 39, 2749–2760. [Google Scholar] [CrossRef]
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Fast Microwave-Assisted Synthesis of Magnetic Molecularly Imprinted Polymer for Sulfamethoxazole. Talanta 2021, 232, 122430. [Google Scholar] [CrossRef] [PubMed]
- Gheybalizadeh, H.; Hejazi, P. Influence of Hydrophilic and Hydrophobic Functional Monomers on the Performance of Magnetic Molecularly Imprinted Polymers for Selective Recognition of Human Insulin. React. Funct. Polym. 2022, 171, 105152. [Google Scholar] [CrossRef]
- Ye, L.; Yoshimatsu, K.; Kolodziej, D.; Da Cruz Francisco, J.; Dey, E.S. Preparation of Molecularly Imprinted Polymers in Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2006, 102, 2863–2867. [Google Scholar] [CrossRef]
- Kubisa, P. Kinetics of Radical Polymerization in Ionic Liquids. Eur. Polym. J. 2020, 133, 109778. [Google Scholar] [CrossRef]
- Fox, D.M.; Awad, W.H.; Gilman, J.W.; Maupin, P.H.; De Long, H.C.; Trulove, P.C. Flammability, Thermal Stability, and Phase Change Characteristics of Several Trialkylimidazolium Salts. Green Chem. 2003, 5, 724–727. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Jutz, F.; Andanson, J.M.; Baiker, A. Ionic Liquids and Dense Carbon Dioxide: A Beneficial Biphasic System for Catalysis. Chem. Rev. 2011, 111, 322–353. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Analytical Applications of Room-Temperature Ionic Liquids: A Review of Recent Efforts. Anal. Chim. Acta 2006, 556, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Silvester, D.S.; Compton, R.G. Electrochemistry in Room Temperature Ionic Liquids: A Review and Some Possible Applications. Z. Phys. Chemie 2006, 220, 1247–1274. [Google Scholar] [CrossRef]
- Kathiresan, M.; Velayutham, D. Ionic Liquids as an Electrolyte for the Electro Synthesis of Organic Compounds. Chem. Commun. 2015, 51, 17499–17516. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Du, Y.; Zhang, G.; Zhao, P.; Lu, J. Electrosynthesis of Polyaniline in Ionic Liquid and Its Electrocatalytic Properties. Front. Chem. China 2006, 1, 345–349. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Atobe, M.; Fuchigami, T. Electropolymerization of Pyrrole in 1-Ethyl-3-Methylimidazolium Trifluoromethanesulfonate Room Temperature Ionic Liquid. Electrochem. Commun. 2002, 4, 881–885. [Google Scholar] [CrossRef]
- Booker, K.; Holdsworth, C.I.; Doherty, C.M.; Hill, A.J.; Bowyer, M.C.; McCluskey, A. Ionic Liquids as Porogens for Molecularly Imprinted Polymers: Propranolol, a Model Study. Org. Biomol. Chem. 2014, 12, 7201–7210. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, Y.; Zhang, Z.; Yang, Y.; Zhai, Y.; Wang, H.; Liu, L.; Hu, J.; Li, L. A New Composite of Graphene and Molecularly Imprinted Polymer Based on Ionic Liquids as Functional Monomer and Cross-Linker for Electrochemical Sensing 6-Benzylaminopurine. Biosens. Bioelectron. 2018, 108, 38–45. [Google Scholar] [CrossRef]
- Xiang, H.; Peng, M.; Li, H.; Peng, S.; Shi, S. High-Capacity Hollow Porous Dummy Molecular Imprinted Polymers Using Ionic Liquid as Functional Monomer for Selective Recognition of Salicylic Acid. J. Pharm. Biomed. Anal. 2017, 133, 75–81. [Google Scholar] [CrossRef]
- Booker, K.; Bowyer, M.C.; Holdsworth, C.I.; McCluskey, A. Efficient Preparation and Improved Sensitivity of Molecularly Imprinted Polymers Using Room Temperature Ionic Liquids. Chem. Commun. 2006, 28, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically Synthesized Polymers in Molecular Imprinting for Chemical Sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef]
- Pietrzyk-Le, A.; Kutner, W.; Chitta, R.; Zandler, M.E.; D’Souza, F.; Sannicolò, F.; Mussini, P.R. Melamine Acoustic Chemosensor Based on Molecularly Imprinted Polymer Film. Anal. Chem. 2009, 81, 10061–10070. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Y.; Liu, Y.; Zhao, F.; Zeng, B. Determination of Patulin Using Dual-Dummy Templates Imprinted Electrochemical Sensor with PtPd Decorated N-Doped Porous Carbon for Amplification. Microchim. Acta 2021, 188, 148. [Google Scholar] [CrossRef]
- McDaniel, J.G.; Son, C.Y.; Yethiraj, A. Ab Initio Force Fields for Organic Anions: Properties of [BMIM][TFSI], [BMIM][FSI], and [BMIM][OTf] Ionic Liquids. J. Phys. Chem. B 2018, 122, 4101–4114. [Google Scholar] [CrossRef]
- Sannicolò, F.; Rizzo, S.; Benincori, T.; Kutner, W.; Noworyta, K.; Sobczak, J.W.; Bonometti, V.; Falciola, L.; Mussini, P.R.; Pierini, M. An Effective Multipurpose Building Block for 3D Electropolymerisation: 2,2′-Bis(2,2′-Bithiophene-5-Yl)-3,3′-Bithianaphthene. Electrochim. Acta 2010, 55, 8352–8364. [Google Scholar] [CrossRef]
- Huynh, T.P.; Pietrzyk-Le, A.; Bikram, K.C.; Noworyta, K.R.; Sobczak, J.W.; Sharma, P.S.; D’Souza, F.; Kutner, W. Electrochemically Synthesized Molecularly Imprinted Polymer of Thiophene Derivatives for Flow-Injection Analysis Determination of Adenosine-5’-Triphosphate (ATP). Biosens. Bioelectron. 2013, 41, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Wojnarowicz, A.; Kelm, A.; Woznicki, P.; Borowicz, P.; Majka, A.; D’Souza, F.; Kutner, W. Chemosensor for Selective Determination of 2,4,6-Trinitrophenol Using a Custom Designed Imprinted Polymer Recognition Unit Cross-Linked to a Fluorophore Transducer. ACS Sens. 2016, 1, 636–639. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Grecchi, S.; Santagostini, L.; Sannicolò, F.; Mussini, P.R. “Inherently Chiral” Thiophene-Based Electrodes at Work: A Screening of Enantioselection Ability toward a Series of Pharmaceutically Relevant Phenolic or Catecholic Amino Acids, Amino Esters, and Amine. Anal. Bioanal. Chem. 2016, 408, 7243–7254. [Google Scholar] [CrossRef]
- Ainslie, R.G.; Gibson, M.K.; Vogel, R.K. MTOR, Autophagy, Aminoacidopathies, and Human Genetic Disorders; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 1. [Google Scholar] [CrossRef]
- Nygaard, G.; Szigetvari, P.D.; Grindheim, A.K.; Ruoff, P.; Martinez, A.; Haavik, J.; Kleppe, R.; Flydal, M.I. Personalized Medicine to Improve Treatment of Dopa-Responsive Dystonia—A Focus on Tyrosine Hydroxylase Deficiency. J. Pers. Med. 2021, 11, 1186. [Google Scholar] [CrossRef]
- Lehnert, H.; Reinstein, D.K.; Strowbridge, B.W.; Wurtman, R.J. Neurochemical and Behavioral Consequences of Acute, Uncontrollable Stress: Effects of Dietary Tyrosine. Brain Res. 1984, 303, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Elsworth, J.D.; Bradberry, C.W.; Roth, R.H. Mesocortical Dopamine Neurons: High Basal Firing Frequency Predicts Tyrosine Dependence of Dopamine Synthesis. J. Neural Transm. 1990, 81, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Der-Avakian, A.; Markou, A. The Neurobiology of Anhedonia and Other Reward-Related Deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Olguin, S.L.; Talledo, J.A.; Kotz, J.E.; Roberts, B.Z.; Nungaray, J.A.; Sprock, J.; Gregg, D.; Bhakta, S.G.; Light, G.A.; et al. Amphetamine Alters an EEG Marker of Reward Processing in Humans and Mice. Psychopharmacology 2022, 239, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Neurauter, G.; Scholl-Bürgi, S.; Haara, A.; Geisler, S.; Mayersbach, P.; Schennach, H.; Fuchs, D. Simultaneous Measurement of Phenylalanine and Tyrosine by High Performance Liquid Chromatography (HPLC) with Fluorescence Detection. Clin. Biochem. 2013, 46, 1848–1851. [Google Scholar] [CrossRef]
- Ishii, Y.; Iijima, M.; Umemura, T.; Nishikawa, A.; Iwasaki, Y.; Ito, R.; Saito, K.; Hirose, M.; Nakazawa, H. Determination of Nitrotyrosine and Tyrosine by High-Performance Liquid Chromatography with Tandem Mass Spectrometry and Immunohistochemical Analysis in Livers of Mice Administered Acetaminophen. J. Pharm. Biomed. Anal. 2006, 41, 1325–1331. [Google Scholar] [CrossRef]
- Li, Y.; Cai, N.; Wang, M.; Na, W.; Shi, F.; Su, X. Fluorometric Detection of Tyrosine and Cysteine Using Graphene Quantum Dots. RSC Adv. 2016, 6, 33197–33204. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Neri, G. Determination of Phenylalanine by a Novel Enzymatic PHD/SPE Biosensor. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Guth, U.; Vonau, W.; Zosel, J. Recent Developments in Electrochemical Sensor Application and Technology—A Review. Meas. Sci. Technol. 2009, 20, 042002. [Google Scholar] [CrossRef]
- Wei, F.; Patel, P.; Liao, W.; Chaudhry, K.; Zhang, L.; Hu, S.; Elashoff, D.; Zhou, H.; Shukla, S.; Shah, F.; et al. Electrochemical Sensor for Multiplex Biomarkers Detection. Clin. Cancer Res. 2009, 15, 4446–4452. [Google Scholar] [CrossRef] [PubMed]
- García-Carmona, L.; González, M.C.; Escarpa, A. Nanomaterial-Based Electrochemical (Bio)-Sensing: One Step Ahead in Diagnostic and Monitoring of Metabolic Rare Diseases. TrAC Trends Anal. Chem. 2019, 118, 29–42. [Google Scholar] [CrossRef]
- Uçar, A.; Aydoğdu Tığ, G.; Er, E. Recent Advances in Two Dimensional Nanomaterial-Based Electrochemical (Bio)Sensing Platforms for Trace-Level Detection of Amino Acids and Pharmaceuticals. TrAC Trends Anal. Chem. 2023, 162, 117027. [Google Scholar] [CrossRef]
- Karazan, Z.M.; Roushani, M. Electrochemical Sensor Based On Molecularly Imprinted Copolymer for Selective and Simultaneous Determination of Ascorbic Acid and Tyrosine. Anal. Bioanal. Chem. Fresearch 2023, 10, 269–278. [Google Scholar]
- Mahdi, N.; Roushani, M.; Karazan, Z.M. Electrochemical Sensor Based on Molecularly Imprinted Copolymer for Selective and Simultaneous Determination of Riboflavin, Dopamine, and L-Tryptophan. J. Mol. Recognit. 2023, 36, e3053. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.; Yu, W.C.; Balram, D.; Patel, A.; Kumar, D.; Yadav, V.K. Nanomolar Detection of Essential Amino Acid in Dairy Products Using a Novel Electrochemical Sensor Based on Zinc Cobaltite Nanoflowers Embedded Porous 3D Reduced Graphene Oxide. Sens. Int. 2024, 5, 100256. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, L.L.; Ni, L.; Xiong, J.M.; He, J.Y.; Zhou, L.D.; Luo, L.; Zhang, Q.H.; Yuan, C.S. Constructing Electrochemical Sensor Using Molecular-Imprinted Polysaccharide for Rapid Identification and Determination of L-Tryptophan in Diet. Food Chem. 2023, 425, 136486. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, O.J.; Mascarenhas, R.J.; Satpati, A.K.; Aiman, L.V.; Mekhalif, Z. Electrocatalytic Oxidation of L-Tyrosine at Carboxylic Acid Functionalized Multi-Walled Carbon Nanotubes Modified Carbon Paste Electrode. Ionics 2016, 22, 405–414. [Google Scholar] [CrossRef]
- Yu, X.Y.; He, J.Y.; Tang, F.; Yu, P.; Wu, L.; Xiao, Z.L.; Sun, L.X.; Cao, Z.; Yu, D. Highly Sensitive Determination of L-Glutamic Acid in Pig Serum with an Enzyme-Free Molecularly Imprinted Polymer on a Carbon-Nanotube Modified Electrode. Anal. Methods 2023, 15, 5589–5597. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent Developments, Characteristics and Potential Applications of Screen-Printed Electrodes in Pharmaceutical and Biological Analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.W.; Xiu, G.; Long, Y.T. Applications of Screen-Printed Electrodes in Current Environmental Analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Kerman, K.; Vestergaard, M.; Tamiya, E. Label-Free Electrical Sensing of Small-Molecule Inhibition on Tyrosine Phosphorylation. Anal. Chem. 2007, 79, 6881–6885. [Google Scholar] [CrossRef]
- Saraiva, D.P.M.; Braga, D.V.; Bossard, B.; Bertotti, M. Multiple Pulse Amperometry—An Antifouling Approach for Nitrite Determination Using Carbon Fiber Microelectrodes. Molecules 2023, 28, 387. [Google Scholar] [CrossRef]
- René, W.; Lenoble, V.; Chioukh, M.; Branger, C. A Turn-on Fluorescent Ion-Imprinted Polymer for Selective and Reliable Optosensing of Lead in Real Water Samples. Sens. Actuators B Chem. 2020, 319, 128252. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Niu, X.; Zhou, Y.; Liu, D.; Falahati, M.; Du, D.; Lin, Y. Integrating Ionic Liquids with Molecular Imprinting Technology for Biorecognition and Biosensing: A Review. Biosens. Bioelectron. 2020, 149, 111830. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, J.; Cai, G.; Lin, A.; Zheng, W.; Liu, X.; Chen, L.; He, X.; Zhang, Y. Room Temperature Ionic Liquid-Mediated Molecularly Imprinted Polymer Monolith for the Selective Recognition of Quinolones in Pork Samples. J. Sep. Sci. 2010, 33, 3786–3793. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, C.Y.; Wang, X.H.; Huang, Y.P.; Liu, Z.S. Thermoresponsive Ketoprofen-Imprinted Monolith Prepared in Ionic Liquid. Anal. Bioanal. Chem. 2014, 406, 5359–5367. [Google Scholar] [CrossRef]
- Sun, Y.K.; Sun, G.Y.; Jia, M.; Yang, J.; Liu, Z.S.; Huang, Y.P.; Aisa, H.A. Cost-Effective Imprinting to Minimize Consumption of Template in Room-Temperature Ionic Liquid for Fast Purification of Chlorogenic Acid from the Extract of E. Ulmoides Leaves. Anal. Bioanal. Chem. 2019, 411, 1261–1271. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.L.; Ma, L.; Shang, P.P.; Huang, Y.P.; Liu, Z.S. Improving Affinity of β-Cyclodextrin-Based Molecularly Imprinted Polymer Using Room Temperature Ionic Liquid. Eur. Polym. J. 2019, 116, 275–282. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, M.; Liu, W.; Yu, S.; Niu, L.; Li, G.; Li, H.; Liu, W. Electrochemical Sensor Based on Molecularly Imprinted Polymer/Reduced Graphene Oxide Composite for Simultaneous Determination of Uric Acid and Tyrosine. J. Electroanal. Chem. 2018, 813, 75–82. [Google Scholar] [CrossRef]
- Hassanvand, Z.; Jalali, F. Simultaneous Determination of L-DOPA, L-tyrosine and Uric Acid by Cysteic Acid—Modified Glassy Carbon Electrode. Mater. Sci. Eng. C 2019, 98, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, Y.; Lin, L.; Chen, H.; Zhao, M. Au Nanoparticles @metal Organic Framework/Polythionine Loaded with Molecularly Imprinted Polymer Sensor: Preparation, Characterization, and Electrochemical Detection of Tyrosine. J. Electroanal. Chem. 2020, 863, 114052. [Google Scholar] [CrossRef]
- Nasimi, H.; Madsen, J.S.; Zedan, A.H.; Schmedes, A.V.; Malmendal, A.; Osther, P.J.S.; Alatraktchi, F.A.Z. Correlation between Stage of Prostate Cancer and Tyrosine and Tryptophan in Urine Samples Measured Electrochemically. Anal. Biochem. 2022, 649, 114698. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.D.; Stave, U. A Study of Plasma Free Amino Acid Levels. VI. High Plasma Glycine Levels of Some Women. Metabolism 1973, 22, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Ashley, D.V.; Barclay, D.V.; Chauffard, F.A.; Moennoz, D.; Leathwood, P.D. Plasma Amino Acid Responses in Humans to Evening Meals of Differing Nutritional Composition. Am. J. Clin. Nutr. 1982, 36, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Livesey, G. Effects of Ingested Steak and Infused Leucine on Forelimb Metabolism in Man and the Fate of the Carbon Skeletons and Amino Groups of Branched-Chain Amino Acids. Clin. Sci. 1983, 64, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Folmer, P.; Schlatmann, A.; Goren, A.; Austin, S. Amino Acid Metabolism in Muscle and in the Whole Body of Man before and after Ingestion of a Single Mixed Meal. Am. J. Clin. Nutr. 1989, 49, 1203–1210. [Google Scholar] [CrossRef]
- Van Spronsen, F.J.; Van Rijn, M.; Bekhof, J.; Koch, R.; Smit, P.G.A. Phenylketonuria: Tyrosine Supplementation in Phenylalanine-Restricted Diets. Am. J. Clin. Nutr. 2001, 73, 153–157. [Google Scholar] [CrossRef]
- Adnan, M.; Puranik, S. Hypertyrosinemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]





| Cations | Anions |
|---|---|
| 1-alkyl-3-methylimidazolium | halide |
| 1-alkyl-1-methylpyrrolidinium | tetrachloroaluminate |
| 1-alkyl-1-methylpiperidinium | tetrafluoroborate |
| tetraalkylphosphonium | hexafluorophosphate |
| tetraalkylammonium | bis(trifluoromethane sulfonyl)imide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliani, F.; Di Giulio, T.; Grecchi, S.; Benincori, T.; Arnaboldi, S.; Malitesta, C.; Mazzotta, E. Green Synthesis of a Molecularly Imprinted Polymer Based on a Novel Thiophene-Derivative for Electrochemical Sensing. Molecules 2024, 29, 1632. https://doi.org/10.3390/molecules29071632
Gagliani F, Di Giulio T, Grecchi S, Benincori T, Arnaboldi S, Malitesta C, Mazzotta E. Green Synthesis of a Molecularly Imprinted Polymer Based on a Novel Thiophene-Derivative for Electrochemical Sensing. Molecules. 2024; 29(7):1632. https://doi.org/10.3390/molecules29071632
Chicago/Turabian StyleGagliani, Francesco, Tiziano Di Giulio, Sara Grecchi, Tiziana Benincori, Serena Arnaboldi, Cosimino Malitesta, and Elisabetta Mazzotta. 2024. "Green Synthesis of a Molecularly Imprinted Polymer Based on a Novel Thiophene-Derivative for Electrochemical Sensing" Molecules 29, no. 7: 1632. https://doi.org/10.3390/molecules29071632
APA StyleGagliani, F., Di Giulio, T., Grecchi, S., Benincori, T., Arnaboldi, S., Malitesta, C., & Mazzotta, E. (2024). Green Synthesis of a Molecularly Imprinted Polymer Based on a Novel Thiophene-Derivative for Electrochemical Sensing. Molecules, 29(7), 1632. https://doi.org/10.3390/molecules29071632