Microscopic Understanding of Interfacial Performance and Antifoaming Mechanism of REP Type Block Polyether Nonionic Surfactants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Activity and Foaming Properties of REP Type Block Polyether Nonionic Surfactants
2.2. Dynamic Surface Activity of REP Type Block Polyether Nonionic Surfactants
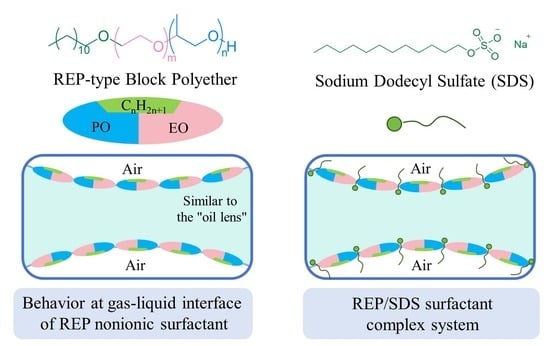
2.3. Molecular Behavior of REP Type Block Polyether Nonionic Surfactants at Gas/Water Interface
2.4. Interfacial Molecular Behavior and Foam Properties of Nonionic/Anionic Surfactant Binary Mixed System
3. Material and Methods
3.1. Materials
3.2. Static Surface Tension
3.3. Foam Performance
3.4. Dynamic Surface Tension
3.5. Molecular Dynamic Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malysa, K.; Lunkenheimer, K. Foams under dynamic conditions. Curr. Opin. Colloid Interface Sci. 2008, 13, 150–162. [Google Scholar] [CrossRef]
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183–184, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liang, Y.; Zhao, Y.; Wang, W.; Hu, X.; Tian, F.; He, Z.; Li, Y.; Liu, T. Preparation of new gel foam and evaluation of its fire extinguishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Ng, E.L.S.; Lau, K.K.; Partoon, B.; Lim, S.F.; Chin, S.Y. Selection criteria for antifoams used in the acid gas sweetening process. Ind. Eng. Chem. Res. 2021, 60, 13438–13462. [Google Scholar] [CrossRef]
- Cevada, E.; Roos, K.; Alvarez, F.; Carlotti, S.; Vázquez, F. High molar mass polyethers as defoamers of heavy crude oil. Fuel 2018, 221, 447–454. [Google Scholar] [CrossRef]
- Yang, P.; Li, L.; Qin, S.; Wu, D.; Peng, Y.; Liu, H.; Peng, X. Effects and mechanisms of foam suppression in food waste anaerobic digester using defoaming agents. Fuel 2023, 342, 127920. [Google Scholar] [CrossRef]
- Vardar-Sukan, F. Foaming: Consequences, prevention and destruction. Biotechnol. Adv. 1998, 16, 913–948. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozdanova, M.V. Foams and antifoams. Adv. Colloid Interface Sci. 2012, 176–177, 1–17. [Google Scholar] [CrossRef]
- Venkatesh, G. Dishwashers: Literature review to summarise the multi-dimensionality of sustainable production and consumption. Sustainability 2022, 14, 10302. [Google Scholar] [CrossRef]
- Alt, T.; Boivin, D.; Altan, M.; Kessler, A.; Schmitz, A.; Stamminger, R. Exploring consumer behaviour in automatic dishwashing: A quantitative investigation of appliance usage in six european countries. Tenside Surfactants Deterg. 2023, 60, 106–116. [Google Scholar] [CrossRef]
- Gooijer, H.; Stamminger, R. Water and energy consumption in domestic laundering worldwide—A review. Tenside Surfactants Deterg. 2016, 53, 402–409. [Google Scholar] [CrossRef]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Ross, S. Profoams and antifoams. Colloids Surf. A Physicochem. Eng. Asp. 1996, 118, 187–192. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of high-intensity ultrasound to improve food processing efficiency: A review. Foods 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Garrett, P.R. Defoaming: Antifoams and mechanical methods. Curr. Opin. Colloid Interface Sci. 2015, 20, 81–91. [Google Scholar] [CrossRef]
- Denkov, N.D. Mechanisms of foam destruction by oil-based antifoams. Langmuir 2004, 20, 9463–9505. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, M.; Zhou, Q.; Wang, B.; Wei, W.; Chen, J. On recycling earth pressure balance shield muck with residual foaming agent: Defoaming and antifoaming investigations. Environ. Sci. Pollut. Res. 2024, 31, 8046–8060. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, X.; Jia, M.; Ma, C.; Li, J.; Shi, M.; Niu, Y. Antifoaming agent for lubricating oil: Preparation, mechanism and application. Molecules 2023, 28, 3152. [Google Scholar] [CrossRef]
- Fauser, H.; von Klitzing, R. Effect of polyelectrolytes on (de)stability of liquid foam films. Soft Matter 2014, 1, 693–6916. [Google Scholar] [CrossRef]
- Cao, X.; Xia, W.; Zhao, T.; Li, X.; Zhang, P.; Qin, X.; Bai, B.; Bian, J. Study on the dual effects of MgCl2 on the characteristics of foams stabilized by sodium dodecyl sulfate. Ind. Eng. Chem. Res. 2023, 62, 16977–16989. [Google Scholar] [CrossRef]
- Arnaudov, L.; Denkov, N.D.; Surcheva, I.; Durbut, P.; Broze, G.; Mehreteab, A. Effect of oily additives on foamability and foam stability. 1. Role of interfacial properties. Langmuir 2001, 17, 6999–7010. [Google Scholar] [CrossRef]
- Denkov, N.D.; Marinova, K.G.; Tcholakova, S.S. Mechanistic understanding of the modes of action of foam control agents. Adv. Colloid Interface Sci. 2014, 206, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Agneta, M.; Zhaomin, L.; Chao, Z.; Gerald, G. Investigating synergism and antagonism of binary mixed surfactants for foam efficiency optimization in high salinity. J. Pet. Sci. Eng. 2019, 175, 489–494. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Li, Y. Interaction between cationic and anionic surfactants: Detergency and foaming properties of mixed systems. J. Surfactants Deterg. 2014, 17, 881–888. [Google Scholar] [CrossRef]
- Chaisalee, R.; Soontravanich, S.; Yanumet, N.; Scamehorn, J.F. Mechanism of antifoam behavior of solutions of nonionic surfactants above the cloud point. J. Surfactants Deterg. 2003, 6, 345–351. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Karlström, G. Clouding of nonionic surfactants. Curr. Opin. Colloid Interface Sci. 2016, 22, 23–29. [Google Scholar] [CrossRef]
- Joshi, K.S.; Jeelani, S.A.K.; Blickenstorfer, C.; Naegeli, I.; Oliviero, C.; Windhab, E.J. Nonionic block copolymer antifoams. Langmuir 2006, 22, 6893–6904. [Google Scholar] [CrossRef] [PubMed]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: Synthesis, novel polymer architectures, and bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Brocas, A.; Mantzaridis, C.; Tunc, D.; Carlotti, S. Polyether synthesis: From activated or metal-free anionic ring-opening polymerization of epoxides to functionalization. Prog. Polym. Sci. 2013, 38, 845–873. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.H.; Finch, J.A. Synthesis and characterization of alkyl, propoxy, ethoxy-based frothers. Miner. Eng. 2016, 95, 66–73. [Google Scholar] [CrossRef]
- Yada, S.; Suzuki, T.; Hashimoto, S.; Yoshimura, T. Adsorption and aggregation properties of homogeneous polyoxypropylene-polyoxyethylene alkyl ether type nonionic surfactants. Langmuir 2017, 33, 3794–3801. [Google Scholar] [CrossRef]
- Yada, S.; Suzuki, T.; Hashimoto, S.; Yoshimura, T. Adsorption dynamics of homogeneous polyoxypropylene-polyoxyethylene alkyl ether nonionic surfactants at the air/water interface. J. Mol. Liq. 2018, 255, 208–214. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Hua, X.Y.; Rosen, M.J. Dynamic surface tension of aqueous surfactant solutions: I. Basic paremeters. J. Colloid Interface Sci. 1988, 124, 652–659. [Google Scholar] [CrossRef]
- Gao, T.; Rosen, M.J. Dynamic surface tension of aqueous surfactant solutions: 7. Physical significance of dynamic parameters and the induction period. J. Colloid Interface Sci. 1995, 172, 242–248. [Google Scholar] [CrossRef]
- Lee, J.; Nikolov, A.; Wasan, D. Effects of micellar structuring and solubilized oil on the kinetic stability of aqueous foams. Ind. Eng. Chem. Res. 2014, 53, 18891–18899. [Google Scholar] [CrossRef]
- Weissenborn, P.K.; Pugh, R.J. Surface tension of aqueous solutions of electrolytes: Relationship with ion hydration, oxygen solubility, and bubble coalescence. J. Colloid Interface Sci. 1996, 184, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Yuet, P.K.; Blankschtein, D. Molecular dynamics simulation study of water surfaces: Comparison of flexible water models. J. Phys. Chem. B 2010, 114, 13786–13795. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, Y.; Qu, G.; Cheng, J.; Xue, C.; Gao, X.; Sun, T.; Ding, W. Molecular dynamics simulation of the aggregation behavior of N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate/sodium dodecyl benzene sulfonate surfactant mixed system at oil/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 73–80. [Google Scholar] [CrossRef]
- Qu, G.; Xue, C.; Zhang, M.; Liang, S.; Han, Y.; Ding, W. Molecular dynamics simulation of sulfobetaine-type zwitterionic surfactants at the decane/water interface: Structure, interfacial properties. J. Dispersion Sci. Technol. 2016, 37, 1710–1717. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]











| Surfactant | CMC (mg/L) | γCMC (mN/m) | Γmax (μmol/m2) | Amin (nm2) |
|---|---|---|---|---|
| AEO-9 | 94.64 | 31.54 | 2.660 | 0.63 |
| C12E5P4 | 63.46 | 30.77 | 2.605 | 0.64 |
| C12E14P8 | 246.69 | 34.85 | 1.190 | 1.40 |
| Surfactant | n | t* (s) | ti (s) | tm (s) | R1/2 (mN m−1 s−1) |
|---|---|---|---|---|---|
| AEO-9 | 0.824 | 0.106 | 6.47 × 10−3 | 1.72 | 185.6 |
| C12E5P4 | 0.668 | 0.152 | 4.83 × 10−3 | 4.75 | 140.8 |
| C12E14P8 | 0.652 | 0.012 | 3.40 × 10−4 | 0.40 | 1643.7 |
| Surfactant | Interface Thickness (nm) | Rg (nm) |
|---|---|---|
| C12E5P4 | 0.76 | 6.21 |
| C12E14P8 | 1.61 | 7.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xue, C.; Ji, D.; Gong, W.; Liu, Y.; Li, Y. Microscopic Understanding of Interfacial Performance and Antifoaming Mechanism of REP Type Block Polyether Nonionic Surfactants. Molecules 2024, 29, 1816. https://doi.org/10.3390/molecules29081816
Zhao Y, Xue C, Ji D, Gong W, Liu Y, Li Y. Microscopic Understanding of Interfacial Performance and Antifoaming Mechanism of REP Type Block Polyether Nonionic Surfactants. Molecules. 2024; 29(8):1816. https://doi.org/10.3390/molecules29081816
Chicago/Turabian StyleZhao, Yifei, Chunlong Xue, Deluo Ji, Weiqian Gong, Yue Liu, and Ying Li. 2024. "Microscopic Understanding of Interfacial Performance and Antifoaming Mechanism of REP Type Block Polyether Nonionic Surfactants" Molecules 29, no. 8: 1816. https://doi.org/10.3390/molecules29081816
APA StyleZhao, Y., Xue, C., Ji, D., Gong, W., Liu, Y., & Li, Y. (2024). Microscopic Understanding of Interfacial Performance and Antifoaming Mechanism of REP Type Block Polyether Nonionic Surfactants. Molecules, 29(8), 1816. https://doi.org/10.3390/molecules29081816