Degradation of Microcystin-LR and RR by a Stenotrophomonas sp. Strain EMS Isolated from Lake Taihu, China
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Isolation and Identification of MC-Degrading Bacteria EMS
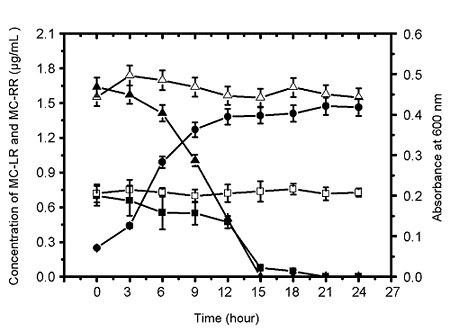
2.1.2. Biodegradation of MC-LR and MC-RR by Strain EMS
2.1.3. Detection of mlrA Gene from MCs-degrading Bacteria Strain EMS
2.2. Discussion
3. Experimental Section
3.1. Extraction of MCs
3.2. Detection and Quantification of MC-LR and MC-RR
3.3. Isolation of Bacterial Strain EMS with the Activity of MCs Degradation
3.4. Identification of the EMS Strain
3.5. Experiment Design of MCs Biodegradation by Strain EMS
3.6. PCR for mlrA and Sequence Analysis
4. Conclusions
Acknowledgments
References and Notes
- Wu, S; Wang, S; Yang, H; Xie, P; Ni, L; Xu, J. Field studies on the environmental factors in controlling microcystin production in the subtropical shallow lakes of the Yangtze River. Bull. Environ. Contam. Toxicol 2008, 80, 329–334. [Google Scholar]
- Yang, H; Xie, P; Xu, J; Zheng, L; Deng, D; Zhou, Q; Wu, S. Seasonal variation of microcystin concentration in Lake Chaohu, a shallow subtropical lake in the People's Republic of China. Bull. Environ. Contam. Toxicol 2006, 77, 367–374. [Google Scholar]
- Xiao, FG; Zhao, XL; Tang, J; Gu, XH; Zhang, JP; Niu, WM. Determination of microcystin-LR in water from Lake Tai, China. Bull. Environ. Contam. Toxicol 2009, 82, 230–233. [Google Scholar]
- Welker, M; von Döhren, H. Cyanobacterial peptides-Nautre’s own combinatorial biosynthesis. FEMS Microbiol. Rev 2006, 30, 530–563. [Google Scholar]
- Zhang, JB; Zheng, Z; Yang, G; Zhao, YF. Degradation of microcystin by gamma irradiation. Nucl. Instr. Meth. Phys. Res. A 2007, 580, 687–689. [Google Scholar]
- Dawsan, RM. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar]
- Briand, JF; Jacquet, S; Bernard, C; Humbert, JF. Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Vet. Res 2003, 34, 361–377. [Google Scholar]
- Žegura, B; Lah, TT; Filipič, M. The role of reactive oxyen species in microcystin-LR-induced DNA damage. Toxicology 2004, 200, 59–68. [Google Scholar]
- Žegura, B; Sedmak, B; Filipič, M. Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 2003, 41, 41–48. [Google Scholar]
- Li, H; Xie, P; Li, G; Hao, L; Xiong, Q. In vivo study on the effects of microcystin extracts on the expression profiles of proto-oncogenes (c-fos, c-jun and c-myc) in liver, kidney and testis of male Wistar rats injected i.v. with toxins. Toxicon 2009, 53, 169–175. [Google Scholar]
- Mezhoud, K; Praseuth, D; Puiseux-Dao, S; François, J; Bernard, C; Edery, M. Global quantitative analysis of protein expression and phosphorylation status in the liver of the medaka fish (Oryzias latipes) exposed to microcystin-LR I. Balneation study. Aquat. Toxicol 2007, 86, 166–175. [Google Scholar]
- Crush, JR; Briggs, LR; Sprosen, JM; Nichols, SN. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol 2008, 23, 246–252. [Google Scholar]
- Järvenpää, S; Lundberg-Niinistö, C; Spoof, L; Sjövall, O; Tyystjärvi, E; Meriluoto, J. Effects of microcystins on broccoli and mustard, and analysis of accumulated toxin by liquid chromatography-mass spectrometry. Toxicon 2007, 49, 865–874. [Google Scholar]
- Máthé, C; M-Hamvas, M; Vasas, G; Surányi, G; Bácsi, I; Beyer, D; Tóth, S; Tímár, M; Borbély, G. Microcystin-LR, a cyanobacterial toxin, induces growth inhibition and histological alterations in common reed (Phragmites australis) plants regenerated from embryogenic calli. New Phytol 2007, 176, 824–835. [Google Scholar]
- Pflugmacher, S; Aulhorn, M; Grimm, B. Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol 2007, 175, 482–489. [Google Scholar]
- Park, HD; Sasaki, Y; Maruyama, T; Yanagisawa, E; Hiraishi, A; Kato, K. Degradation of the cyanobacterial hepatotoxin microcystin by a new bacterium isolated from a hypertrophic lake. Environ. Toxicol 2001, 16, 337–343. [Google Scholar]
- Mao, XW; Xu, F; Xu, B; Gao, Y. Changes of water quality and eutrophication in Taihu Lake. Water Resour. Prot 2009, 25, 48–51. [Google Scholar]
- Dong, X; Bennion, H; Battarbee, R; Yang, X; Yang, H; Liu, E. Tracking eutrophication in Taihu Lake using the diatom record: potential and problems. J. Paleolimnol 2008, 40, 413–429. [Google Scholar]
- Liu, W; Qiu, R. Water eutrophication in China and the combating strategies. J. Chem. Technol. Biotechnol 2007, 82, 781–786. [Google Scholar]
- Duan, H; Ma, R; Xu, X; Kong, F; Zhang, S; Kong, W; Hao, J; Shang, L. Two-decade reconstruction of algal blooms in China's Lake Taihu. Environ. Sci. Technol 2009, 43, 3522–3528. [Google Scholar]
- Guidelines for Drinking-Water Quality, 3rd ed; WHO (World Health Organization): Geneva, Switzerland, 2004; Volume 1.
- Standards for drinking water quality (GB 5749-2006); MHPRC, (Ministry of Health of the People’s Republic of China): Beijing, China, 2006.
- Edwards, C; Lawton, LA. Bioremediation of cyanotoxins. In Advances in Applied Microbiology; Laskin, AI, Gadd, GM, Sariaslani, S, Eds.; Academic Press: New York, NY, USA, 2009; Volume 67, pp. 109–129. [Google Scholar]
- Bourne, DG; Blakeley, RL; Riddles, P; Jones, GJ. Biodegradation of the cyanobacterial toxin microcystin LR in natural water and biologically active slow sand filters. Water Res 2006, 40, 1294–1302. [Google Scholar]
- Ho, L; Meyn, T; Keegan, A; Hoefel, D; Brooks, J; Saint, CP; Newcombe, G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res 2006, 40, 768–774. [Google Scholar]
- Hu, LB; Yang, JD; Zhou, W; Yin, YF; Chen, J; Shi, ZQ. Isolation of a Methylobacillus sp. that degrades of microcyctin toxins associated with cyanobacteria. N. Biotechnol 2009, 26, 205–211. [Google Scholar]
- Bourne, DG; Jones, GJ; Blakeley, RL; Jones, A; Negri, AP; Riddles, P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Appl. Environ. Microbiol 1996, 62, 4086–4094. [Google Scholar]
- Satio, T; Okano, K; Park, HD; Itayama, T; Inamori, Y; Neilan, BA; Burns, BP; Sugiura, N. Detection and sequencing of the microcystin LR degrading gene, mlrA, from new bacteria isolated from Japanese lakes. FEMS Microbiol. Lett 2003, 229, 271–276. [Google Scholar]
- Harada, KI; Imanishi, S; Kato, H; Mizuno, M; Ito, E; Tsuji, K. Isolation of Adda from microcystin-LR by microbial degradation. Toxicon 2004, 44, 107–109. [Google Scholar]
- Edwards, C; Graham, D; Fowler, N; Lawton, LA. Biodegradation of microcystins and nodularin in freshwaters. Chemosphere 2008, 73, 1315–1321. [Google Scholar]
- Ho, L; Gaudieux, AL; Fanok, S; Newcombe, G; Humpage, AR. Bacterial degradation of microcystin toxins in drinking water eliminates their toxicity. Toxicon 2007, 50, 438–441. [Google Scholar]
- Lemes, GA; Kersanach, R; Pinto, LS; Dellagostin, OA; Yunes, JS; Matthiensen, A. Biodegradation of microcystins by aquatic Burkholderia sp. from a South Brazilian coastal lagoon. Ecotoxicol. Environ. Saf 2008, 69, 358–365. [Google Scholar]
- Manage, PM; Edwards, C; Singh, BK; Lawton, LA. Isolation and identification of novel microcystin-degrading bacteria. Appl. Environ. Microbiol 2009, 75, 6924–6928. [Google Scholar]
- Bourne, DG; Riddles, P; Jones, GJ; Smith, W; Blakeley, RL. Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environ. Toxicol 2001, 16, 523–534. [Google Scholar]
- Huan, HL; Han, L; Li, JH; Wang, YP. Isolation and identification of five microcystin degrading bacterial strains. J. Lake Sci 2006, 18, 184–188. [Google Scholar]
- Liu, HY; Huan, HL; Wang, YW; Li, JH; Zhou, YM. Molecular identification of a microcystin-degrading bacteria strain S3 and its biodegradation of microcystin. Acta Sci. Circumst 2007, 27, 1145–1150. [Google Scholar]
- Müller, TA; Byrde, SM; Werlen, C; Meer, JR; Kohler, HE. Genetic analysis of phenoxyalkanoic acid degradation in Sphingomonas herbicidovorans MH. Appl. Environ. Microbiol 2004, 70, 6066–6075. [Google Scholar]
- Ghosh, D; Roy, K; Srinivasan, V; Mueller, T; Tuovinen, OH; Sublette, K; Peacock, A; Radosevich, M. In-situ enrichment and analysis of atrazine-degradation microbial communities using atrazine-containing porous beads. Soil Biol. Biochem 2009, 41, 1331–1334. [Google Scholar]
- Satio, T; Sugiura, N; Itayama, T; Inamori, Y; Matsumura, M. Degradation characteristics of microcystins by isolated bacteria from Lake Kasumigaura. J. Water Suppl. Res. Technol. AQUA 2003, 52, 13–18. [Google Scholar]
- Jones, GJ; Bourne, DG; Blakeley, RL; Doelle, H. Degradation of the cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat. Toxins 1994, 2, 228–235. [Google Scholar]
- Sedmak, B; Carmeli, S; Eleršek, T. “Non-toxic” cyclic peptides induce lysis of cyanobacteria – an effective cell population density control mechanism in cyanobacterial blooms. Microb. Ecol 2008, 56, 201–209. [Google Scholar]
- Takenaka, S; Watanable, MF. Microcystin LR degradation by Pseudomonas aeruginosa alkaline protease. Chemosphere 1997, 34, 749–757. [Google Scholar]
- Tsuji, K; Asakawa, M; Yojiro, A; Sumino, T; Harada, KI. Degradation of microcystins using immobilized microorganism isolated in a neutrophic lake. Chemosphere 2006, 65, 117–124. [Google Scholar]
- Valeria, AM; Ricardo, EJ; Stephan, P; Alberto, WD. Degradation of microcystin-RR by Sphingomonas sp. CBA4 isolated from San Roque reservoir (Córdoba–Argentina). Biodegradation 2006, 17, 447–455. [Google Scholar]
- Christiansen, G; Fastner, J; Erhard, M; Börner, T; Dittmann, E. Microcystin biosynthesis in Planktothrix: genes, evolution, and manipulation. J. Bacteriol 2003, 185, 564–572. [Google Scholar]
- Ureña, JM; Merlos-Suárez, A; Baselga, J; Arribas, J. The cytoplasmic carboxy-terminal amino acid determines the subcellular localization of proTGF-(alpha) and membrane type matrix metalloprotease (MT1-MMP). J. Cell Sci 1999, 112, 773–784. [Google Scholar]
- Sun, X; Liu, X; Guo, S; Huang, B; Li, W; Wang, X; Lin, J; Tang, K. Cloning and analysis of a novel conserved membrane zinc metalloprotease family from Solanum surattense. Russ. J. Plant Physiol 2007, 54, 63–73. [Google Scholar]
- Lane, DJ; Pace, B; Olsen, GJ; Sogin, ML; Pace, NR. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 882, 6955–6959. [Google Scholar]
- Larkin, MA; Blackshields, G; Brown, NP; Chenna, R; McGettigan, PA; McWilliam, H; Valentin, F; Wallace, IM; Wilm, A; Lopez, R; Thompson, JD; Gibson, TJ; Higgins, DG. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
- Kumar, S; Dudley, J; Nei, M; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform 2008, 9, 299–306. [Google Scholar]









| Bacterial strains | GeneBank accession number in NCBI | Similarity with mlrA-EMS | Reference |
|---|---|---|---|
| Sphingopyxis sp. C-1 | AB468058 | 98% | Direct submisson |
| Sphingopyxis sp. LH21 | DQ112243 | 98% | Direct submisson |
| Sphingomonas sp. ACM-3962 | AF411068 | 92% | [34] |
| Sphingomonas sp. MD-1 | AB114202 | 91% | [28] |
| Sphingomonas sp. Y2 | AB114203 | 83% | [28] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Hu, L.B.; Zhou, W.; Yan, S.H.; Yang, J.D.; Xue, Y.F.; Shi, Z.Q. Degradation of Microcystin-LR and RR by a Stenotrophomonas sp. Strain EMS Isolated from Lake Taihu, China. Int. J. Mol. Sci. 2010, 11, 896-911. https://doi.org/10.3390/ijms11030896
Chen J, Hu LB, Zhou W, Yan SH, Yang JD, Xue YF, Shi ZQ. Degradation of Microcystin-LR and RR by a Stenotrophomonas sp. Strain EMS Isolated from Lake Taihu, China. International Journal of Molecular Sciences. 2010; 11(3):896-911. https://doi.org/10.3390/ijms11030896
Chicago/Turabian StyleChen, Jian, Liang Bin Hu, Wei Zhou, Shao Hua Yan, Jing Dong Yang, Yan Feng Xue, and Zhi Qi Shi. 2010. "Degradation of Microcystin-LR and RR by a Stenotrophomonas sp. Strain EMS Isolated from Lake Taihu, China" International Journal of Molecular Sciences 11, no. 3: 896-911. https://doi.org/10.3390/ijms11030896
APA StyleChen, J., Hu, L. B., Zhou, W., Yan, S. H., Yang, J. D., Xue, Y. F., & Shi, Z. Q. (2010). Degradation of Microcystin-LR and RR by a Stenotrophomonas sp. Strain EMS Isolated from Lake Taihu, China. International Journal of Molecular Sciences, 11(3), 896-911. https://doi.org/10.3390/ijms11030896