Stem Cell-Based Neuroprotective and Neurorestorative Strategies
Abstract
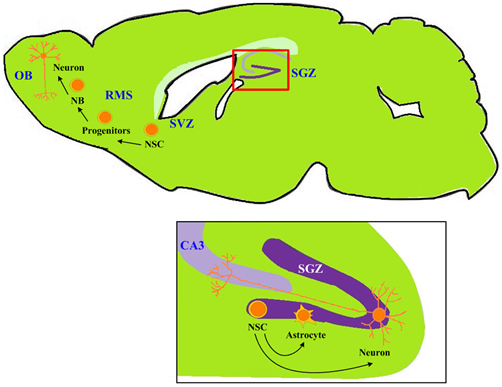
:1. Introduction
2. Depression and Neurogenesis: Evidence from Neural Stem Cells
Antidepressant and Neuroprotection: Interaction with Neural Stem Cells
3. Diseases of Central Nervous System and Neural Stem Cells – Stem Cell Therapy and the Development of New Target Drug
3.1. Parkinson’s Disease
3.2. Ischemic Stroke
3.3. The Hope and Hype of Induced Pluripotent Stem Cells in Cell Replacement Therapy of Neurological Diseases
4. Diet and Neurogenesis
5. Neural Stem Cell, Chinese Herbs, and New Drug Screening
6. Conclusions
Acknowledgments
References
- Gage, FH. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar]
- Alvarez-Buylla, A; Lim, DA. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar]
- Ma, DK; Bonaguidi, MA; Ming, GL; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res 2009, 19, 672–682. [Google Scholar]
- Ma, DK; Ming, GL; Song, H. Glial influences on neural stem cell development: Cellular niches for adult neurogenesis. Curr. Opin. Neurobiol 2005, 15, 514–520. [Google Scholar]
- Taupin, P; Gage, FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J. Neurosci. Res 2002, 69, 745–749. [Google Scholar]
- Gurvits, TV; Shenton, ME; Hokama, H; Ohta, H; Lasko, NB; Gilbertson, MW; Orr, SP; Kikinis, R; Jolesz, FA; McCarley, RW; et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol. Psychiatry 1996, 40, 1091–1099. [Google Scholar]
- Wong, ML; Licinio, J. Research and treatment approaches to depression. Nat. Rev. Neurosci 2001, 2, 343–351. [Google Scholar]
- Moser, MB; Moser, EI. Functional differentiation in the hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar]
- Kempermann, G; Krebs, J; Fabel, K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry 2008, 21, 290–295. [Google Scholar]
- Sapolsky, RM. Why stress is bad for your brain. Science 1996, 273, 749–750. [Google Scholar]
- Sahay, A; Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci 2007, 10, 1110–1115. [Google Scholar]
- Videbech, P; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar]
- Campbell, S; Marriott, M; Nahmias, C; MacQueen, GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar]
- Santarelli, L; Saxe, M; Gross, C; Surget, A; Battaglia, F; Dulawa, S; Weisstaub, N; Lee, J; Duman, R; Arancio, O; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar]
- Czeh, B; Lucassen, PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur. Arch Psychiatry Clin Neurosci 2007, 257, 250–260. [Google Scholar]
- McEwen, BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005, 54, 20–23. [Google Scholar]
- Airan, RD; Meltzer, LA; Roy, M; Gong, Y; Chen, H; Deisseroth, K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 2007, 317, 819–823. [Google Scholar]
- Meshi, D; Drew, MR; Saxe, M; Ansorge, MS; David, D; Santarelli, L; Malapani, C; Moore, H; Hen, R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat. Neurosci 2006, 9, 729–731. [Google Scholar]
- Saxe, MD; Battaglia, F; Wang, JW; Malleret, G; David, DJ; Monckton, JE; Garcia, AD; Sofroniew, MV; Kandel, ER; Santarelli, L; et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA 2006, 103, 17501–17506. [Google Scholar]
- Thomas, RM; Peterson, DA. Even neural stem cells get the blues: Evidence for a molecular link between modulation of adult neurogenesis and depression. Gene Expr 2008, 14, 183–193. [Google Scholar]
- Li, YF; Zhang, YZ; Liu, YQ; Wang, HL; Yuan, L; Luo, ZP. Moclobemide up-regulates proliferation of hippocampal progenitor cells in chronically stressed mice. Acta. Pharmacol. Sin 2004, 25, 1408–1412. [Google Scholar]
- Bonnet, U; Leniger, T; Wiemann, M. Moclobemide reduces intracellular pH and neuronal activity of CA3 neurones in guinea-pig hippocampal slices-implication for its neuroprotective properties. Neuropharmacology 2000, 39, 2067–2074. [Google Scholar]
- Lee, HJ; Kim, JW; Yim, SV; Kim, MJ; Kim, SA; Kim, YJ; Kim, CJ; Chung, JH. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol. Psychiatry 2001, 6, 725–728. [Google Scholar]
- Chiou, SH; Sheu, BC; Chang, WC; Huang, SC; Hong-Nerng, H. Current concepts of tumor-infiltrating lymphocytes in human malignancies. J. Reprod. Immunol 2005, 67, 35–50. [Google Scholar]
- Chen, H; Pandey, GN; Dwivedi, Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport 2006, 17, 863–867. [Google Scholar]
- Goldman, S. Stem and progenitor cell-based therapy of the human central nervous system. Nat. Biotechnol 2005, 23, 862–871. [Google Scholar]
- Chen, SJ; Kao, CL; Chang, YL; Yen, CJ; Shui, JW; Chien, CS; Chen, IL; Tsai, TH; Ku, HH; Chiou, SH. Antidepressant administration modulates neural stem cell survival and serotoninergic differentiation through bcl-2. Curr. Neurovasc. Res 2007, 4, 19–29. [Google Scholar]
- Chiou, SH; Chen, SJ; Peng, CH; Chang, YL; Ku, HH; Hsu, WM; Ho, LL; Lee, CH. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem. Biophys. Res. Commun 2006, 343, 391–400. [Google Scholar]
- Chiou, SH; Ku, HH; Tsai, TH; Lin, HL; Chen, LH; Chien, CS; Ho, LL; Lee, CH; Chang, YL. Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br. J. Pharmacol 2006, 148, 587–598. [Google Scholar]
- Peng, CH; Chiou, SH; Chen, SJ; Chou, YC; Ku, HH; Cheng, CK; Yen, CJ; Tsai, TH; Chang, YL; Kao, CL. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur. Neuropsychopharmacol 2008, 18, 128–140. [Google Scholar]
- Huang, CJ; Cheng, HH; Chou, CT; Kuo, CC; Lu, YC; Tseng, LL; Chu, ST; Hsu, SS; Wang, JL; Lin, KL; et al. Desipramine-induced Ca2+ movement and cytotoxicity in PC3 human prostate cancer cells. Toxicol. In Vitro 2007, 21, 449–456. [Google Scholar]
- Irmler, M; Thome, M; Hahne, M; Schneider, P; Hofmann, K; Steiner, V; Bodmer, JL; Schroter, M; Burns, K; Mattmann, C; et al. Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388, 190–195. [Google Scholar]
- Schulze-Bergkamen, H; Brenner, D; Krueger, A; Suess, D; Fas, SC; Frey, CR; Dax, A; Zink, D; Buchler, P; Muller, M; et al. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology 2004, 39, 645–654. [Google Scholar]
- Xu, H; Chen, Z; He, J; Haimanot, S; Li, X; Dyck, L; Li, XM. Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 2006, 16, 551–559. [Google Scholar]
- Hayley, S; Poulter, MO; Merali, Z; Anisman, H. The pathogenesis of clinical depression: Stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 2005, 135, 659–678. [Google Scholar]
- Manji, HK; Chen, G. PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol. Psychiatry 2002, 7, S46–S56. [Google Scholar]
- Shirayama, Y; Chen, A; Nakagawa, S; Russell, DS; Duman, RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002, 22, 3251–3261. [Google Scholar]
- Einat, H; Manji, HK. Cellular plasticity cascades: Genes-to-behavior pathways in animal models of bipolar disorder. Biol. Psychiatry 2006, 59, 1160–1171. [Google Scholar]
- Larsson, E; Nanobashvili, A; Kokaia, Z; Lindvall, O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J. Cereb. Blood Flow Metab 1999, 19, 1220–1228. [Google Scholar]
- Kokaia, Z; Nawa, H; Uchino, H; Elmer, E; Kokaia, M; Carnahan, J; Smith, ML; Siesjo, BK; Lindvall, O. Regional brain-derived neurotrophic factor mRNA and protein levels following transient forebrain ischemia in the rat. Brain. Res. Mol. Brain. Res 1996, 38, 139–144. [Google Scholar]
- Zhang, ZG; Chopp, M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol 2009, 8, 491–500. [Google Scholar]
- Lindvall, O; Kokaia, Z; Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med 2004, 10, S42–S50. [Google Scholar]
- Samii, A; Nutt, JG; Ransom, BR. Parkinson's disease. Lancet 2004, 363, 1783–1793. [Google Scholar]
- Fearnley, JM; Lees, AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar]
- Lindvall, O; Hagell, P. Clinical observations after neural transplantation in Parkinson's disease. Prog. Brain Res 2000, 127, 299–320. [Google Scholar]
- Kordower, JH; Freeman, TB; Snow, BJ; Vingerhoets, FJ; Mufson, EJ; Sanberg, PR; Hauser, RA; Smith, DA; Nauert, GM; Perl, DP; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N. Engl. J. Med 1995, 332, 1118–1124. [Google Scholar]
- Freed, CR; Greene, PE; Breeze, RE; Tsai, WY; DuMouchel, W; Kao, R; Dillon, S; Winfield, H; Culver, S; Trojanowski, JQ; et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med 2001, 344, 710–719. [Google Scholar]
- Olanow, CW; Goetz, CG; Kordower, JH; Stoessl, AJ; Sossi, V; Brin, MF; Shannon, KM; Nauert, GM; Perl, DP; Godbold, J; et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol 2003, 54, 403–414. [Google Scholar]
- Hagell, P; Piccini, P; Bjorklund, A; Brundin, P; Rehncrona, S; Widner, H; Crabb, L; Pavese, N; Oertel, WH; Quinn, N; et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat. Neurosci 2002, 5, 627–628. [Google Scholar]
- Hedlund, E; Perlmann, T. Neuronal cell replacement in Parkinson's disease. J. Intern. Med 2009, 266, 358–371. [Google Scholar]
- Yang, D; Zhang, ZJ; Oldenburg, M; Ayala, M; Zhang, SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 2008, 26, 55–63. [Google Scholar]
- Glavaski-Joksimovic, A; Virag, T; Chang, QA; West, NC; Mangatu, TA; McGrogan, MP; Dugich-Djordjevic, M; Bohn, MC. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant 2009, 18, 801–814. [Google Scholar]
- Adams, HP, Jr; del Zoppo, G; Alberts, MJ; Bhatt, DL; Brass, L; Furlan, A; Grubb, RL; Higashida, RT; Jauch, EC; Kidwell, C; et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007, 38, 1655–1711. [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N. Engl. J. Med 1995, 333, 1581–1587.
- Arvidsson, A; Collin, T; Kirik, D; Kokaia, Z; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med 2002, 8, 963–970. [Google Scholar]
- Farooq, MU; Naravetla, B; Moore, PW; Majid, A; Gupta, R; Kassab, MY. Role of sildenafil in neurological disorders. Clin. Neuropharmacol 2008, 31, 353–362. [Google Scholar]
- Chopp, M; Li, Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol 2002, 1, 92–100. [Google Scholar]
- Takahashi, K; Yasuhara, T; Shingo, T; Muraoka, K; Kameda, M; Takeuchi, A; Yano, A; Kurozumi, K; Agari, T; Miyoshi, Y; et al. Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res 2008, 1234, 172–182. [Google Scholar]
- Yanagisawa, D; Qi, M; Kim, DH; Kitamura, Y; Inden, M; Tsuchiya, D; Takata, K; Taniguchi, T; Yoshimoto, K; Shimohama, S; et al. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci. Lett 2006, 407, 74–79. [Google Scholar]
- Okita, K; Ichisaka, T; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar]
- Park, IH; Zhao, R; West, JA; Yabuuchi, A; Huo, H; Ince, TA; Lerou, PH; Lensch, MW; Daley, GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146. [Google Scholar]
- Takahashi, K; Tanabe, K; Ohnuki, M; Narita, M; Ichisaka, T; Tomoda, K; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar]
- Yu, J; Vodyanik, MA; Smuga-Otto, K; Antosiewicz-Bourget, J; Frane, JL; Tian, S; Nie, J; Jonsdottir, GA; Ruotti, V; Stewart, R; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar]
- Takahashi, K; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar]
- Kao, CL; Tai, LK; Chiou, SH; Chen, YJ; Lee, KH; Chou, SJ; Chang, YL; Chang, CM; Chen, SJ; Ku, HH; et al. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev 2010, 19, 247–258. [Google Scholar]
- Wernig, M; Zhao, JP; Pruszak, J; Hedlund, E; Fu, D; Soldner, F; Broccoli, V; Constantine-Paton, M; Isacson, O; Jaenisch, R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc. Natl. Acad. Sci. USA 2008, 105, 5856–5861. [Google Scholar]
- Chen, SJ; Chang, CM; Tsai, SK; Chang, YL; Chou, SJ; Huang, SS; Tai, LK; Chen, YC; Ku, HH; Li, HY; et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev 2010. [Google Scholar]
- Yamanaka, S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif 2008, 41, S51–S56. [Google Scholar]
- Bjorklund, LM; Sanchez-Pernaute, R; Chung, S; Andersson, T; Chen, IY; McNaught, KS; Brownell, AL; Jenkins, BG; Wahlestedt, C; Kim, KS; et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl. Acad. Sci. USA 2002, 99, 2344–2349. [Google Scholar]
- Erdo, F; Buhrle, C; Blunk, J; Hoehn, M; Xia, Y; Fleischmann, B; Focking, M; Kustermann, E; Kolossov, E; Hescheler, J; et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J. Cereb. Blood Flow Metab 2003, 23, 780–785. [Google Scholar]
- Hedlund, E; Pruszak, J; Ferree, A; Vinuela, A; Hong, S; Isacson, O; Kim, KS. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells 2007, 25, 1126–1135. [Google Scholar]
- Pruszak, J; Sonntag, KC; Aung, MH; Sanchez-Pernaute, R; Isacson, O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells 2007, 25, 2257–2268. [Google Scholar]
- Roy, NS; Cleren, C; Singh, SK; Yang, L; Beal, MF; Goldman, SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med 2006, 12, 1259–1268. [Google Scholar]
- Brederlau, A; Correia, AS; Anisimov, SV; Elmi, M; Paul, G; Roybon, L; Morizane, A; Bergquist, F; Riebe, I; Nannmark, U; et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 2006, 24, 1433–1440. [Google Scholar]
- Hedlund, E; Pruszak, J; Lardaro, T; Ludwig, W; Vinuela, A; Kim, KS; Isacson, O. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells 2008, 26, 1526–1536. [Google Scholar]
- Chung, S; Shin, BS; Hedlund, E; Pruszak, J; Ferree, A; Kang, UJ; Isacson, O; Kim, KS. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J. Neurochem 2006, 97, 1467–1480. [Google Scholar]
- Guillaume, DJ; Johnson, MA; Li, XJ; Zhang, SC. Human embryonic stem cell-derived neural precursors develop into neurons and integrate into the host brain. J. Neurosci. Res 2006, 84, 1165–1176. [Google Scholar]
- Tabar, V; Panagiotakos, G; Greenberg, ED; Chan, BK; Sadelain, M; Gutin, PH; Studer, L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat. Biotechnol 2005, 23, 601–606. [Google Scholar]
- Seminatore, C; Polentes, J; Ellman, D; Kozubenko, N; Itier, V; Tine, S; Tritschler, L; Brenot, M; Guidou, E; Blondeau, J; et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke 2010, 41, 153–159. [Google Scholar]
- Chiou, SH; Huang, CW. Docosahexaenoic acid, teratoma formation and dopaminergic differentiation in iPS cells in parkinson disease-like rats. 2009; unpublished work. [Google Scholar]
- Stangl, D; Thuret, S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr 2009, 4, 271–282. [Google Scholar]
- Lotito, SB; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic Biol. Med 2006, 41, 1727–1746. [Google Scholar]
- An, L; Zhang, YZ; Yu, NJ; Liu, XM; Zhao, N; Yuan, L; Chen, HX; Li, YF. The total flavonoids extracted from Xiaobuxin-Tang up-regulate the decreased hippocampal neurogenesis and neurotrophic molecules expression in chronically stressed rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 32, 1484–1490. [Google Scholar]
- Gong, X; Sucher, NJ. Stroke therapy in traditional Chinese medicine (TCM): Prospects for drug discovery and development. Trends. Pharmacol. Sci 1999, 20, 191–196. [Google Scholar]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer. Treat. Rev 2009, 35, 57–68. [Google Scholar]
- Newman, DJ; Cragg, GM; Snader, KM. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Procol 2003, 66, 1022–1037. [Google Scholar]
- Lee, H; Kim, YO; Kim, H; Kim, SY; Noh, HS; Kang, SS; Cho, GJ; Choi, WS; Suk, K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB. J 2003, 17, 1943–1944. [Google Scholar]
- Tan, Z. Neural protection by naturopathic compounds-an example of tetramethylpyrazine from retina to brain. J. Ocul. Biol. Dis. Infor 2009, 2, 57–64. [Google Scholar]
- Fan, LH; Wang, KZ; Cheng, B; Wang, CS; Dang, XQ. Anti-apoptotic and neuroprotective effects of Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci 2006, 7, 48. [Google Scholar]
- Kao, TK; Ou, YC; Kuo, JS; Chen, WY; Liao, SL; Wu, CW; Chen, CJ; Ling, NN; Zhang, YH; Peng, WH. Neuroprotection by tetramethylpyrazine against ischemic brain injury in rats. Neurochem. Int 2006, 48, 166–176. [Google Scholar]
- Ni, JW; Matsumoto, K; Watanabe, H. Tetramethylpyrazine improves spatial cognitive impairment induced by permanent occlusion of bilateral common carotid arteries or scopolamine in rats. Jpn. J. Pharmacol 1995, 67, 137–141. [Google Scholar]
- Zhang, C; Wang, SZ; Zuo, PP; Cui, X; Cai, J. Protective effect of tetramethylpyrazine on learning and memory function in D-galactose-lesioned mice. Chin. Med. Sci. J 2004, 19, 180–184. [Google Scholar]
- Grzanna, R; Phan, P; Polotsky, A; Lindmark, L; Frondoza, CG. Ginger extract inhibits beta-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes. J. Altern. Complement. Med 2004, 10, 1009–1013. [Google Scholar]
- Lim, WC; Seo, JM; Lee, CI; Pyo, HB; Lee, BC. Stimulative and sedative effects of essential oils upon inhalation in mice. Arch. Pharm. Res 2005, 28, 770–774. [Google Scholar]
- Xu, Q; Yi, LT; Pan, Y; Wang, X; Li, YC; Li, JM; Wang, CP; Kong, LD. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 715–725. [Google Scholar]
- Chiou, SH; Lu, SW. Effect of chiese herb medicine on cellular, biochemical and animal models of depression. 2009; unpublished work. [Google Scholar]

© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hung, C.-W.; Liou, Y.-J.; Lu, S.-W.; Tseng, L.-M.; Kao, C.-L.; Chen, S.-J.; Chiou, S.-H.; Chang, C.-J. Stem Cell-Based Neuroprotective and Neurorestorative Strategies. Int. J. Mol. Sci. 2010, 11, 2039-2055. https://doi.org/10.3390/ijms11052039
Hung C-W, Liou Y-J, Lu S-W, Tseng L-M, Kao C-L, Chen S-J, Chiou S-H, Chang C-J. Stem Cell-Based Neuroprotective and Neurorestorative Strategies. International Journal of Molecular Sciences. 2010; 11(5):2039-2055. https://doi.org/10.3390/ijms11052039
Chicago/Turabian StyleHung, Chia-Wei, Ying-Jay Liou, Shao-Wei Lu, Ling-Ming Tseng, Chung-Lan Kao, Shih-Jen Chen, Shih-Hwa Chiou, and Charn-Jung Chang. 2010. "Stem Cell-Based Neuroprotective and Neurorestorative Strategies" International Journal of Molecular Sciences 11, no. 5: 2039-2055. https://doi.org/10.3390/ijms11052039
APA StyleHung, C. -W., Liou, Y. -J., Lu, S. -W., Tseng, L. -M., Kao, C. -L., Chen, S. -J., Chiou, S. -H., & Chang, C. -J. (2010). Stem Cell-Based Neuroprotective and Neurorestorative Strategies. International Journal of Molecular Sciences, 11(5), 2039-2055. https://doi.org/10.3390/ijms11052039