Effect of 2-Hydroxypropyl-β-cyclodextrin on Solubility of Sparingly Soluble Drug Derivatives of Anthranilic Acid
Abstract
:1. Introduction
2. Results and Discussion
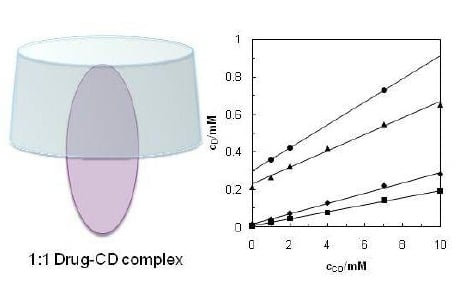
2.1. Solvent Effect and Solution Equilibrium
2.2. Solvent Effect and Solution Equilibrium
3. Experimental Section
3.1. Materials and Instruments
3.2. Preparation of Solutions
4. Conclusions
Acknowledgments
References
- Connors, KA. The stability of cyclodextrin complexes in solution. Chem. Rev 1997, 97, 1325–1357. [Google Scholar]
- De Valle, EMM. Cyclodextrins and their uses: A review. Process Biochem 2004, 39, 1033–1046. [Google Scholar]
- Brewster, ME; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev 2007, 59, 645–666. [Google Scholar]
- Zielenkiewicz, W; Terekhova, IV; Koźbiał, M; Poznański, J; Kumeev, RS. Inclusion of menadione with cyclodextrins studied by calorimetry and spectroscopic methods. J. Phys. Org. Chem 2007, 20, 656–661. [Google Scholar]
- Radi, A-E; Eissa, S. Voltammetric and spectrophotometric study on the complexation of glibenclamide with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem 2010, 68, 417–421. [Google Scholar]
- Popović, G; Čakar, M. The effect of β-cyclodextrin and pH on bifonazole hydrosolubility. J. Serb. Chem. Soc 2004, 69, 225–231. [Google Scholar]
- Tirucherai, GS; Mitra, AK. Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS Pharm. Sci. Tech 2003, 4, 124–135. [Google Scholar]
- Al Omari, AA; Al Omari, MM; Badwan, AA; Al-Sou’od, KA. Effect of cyclodextrins on the solubility and stability of candesartan cilexetil in solution and solid state. J. Pharm. Biomed. Analys 2011, 54, 503–509. [Google Scholar]
- Loftsson, T; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm 2007, 329, 1–11. [Google Scholar]
- Lavecchia, R; Zuorro, A. Experimental study of the inclusion of triclosan in hydroxypropyl-β-cyclodextrins. Chem. Eng. Trans 2009, 17, 1083–1087. [Google Scholar]
- Esclusa-Díaz, MT; Torres-Labandeira, JJ; Kata, M; Vila-Jato, JL. Inclusion complexation of glibenclamide with 2-hydroxypropyl-β-cyclodextrin in solution and in solid state. Eur. J. Pharm. Sci 1994, 1, 291–296. [Google Scholar]
- Taraszewska, J; Migut, K; Koźbiał, M. Complexation of flutamide by native and modified cyclodextrins. J. Phys. Org. Chem 2003, 16, 121–126. [Google Scholar]
- Taraszewska, J; Koźbiał, M. Complexation of ketoconazole by native and modified cyclodextrins. J. Inc. Phenom. Macrom. Chem 2005, 53, 155–161. [Google Scholar]
- Astray, G; Mejuto, JC; Morales, J; Rial-Otero, R; Simal-Gándara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in food. Food Res. Intern 2010, 43, 1212–1218. [Google Scholar]
- Astray, G; González-Barreiro, C; Mejuto, JC; Rial-Otero, R; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll 2009, 23, 1631–1640. [Google Scholar]
- González-Barreiro, C; Rial-Otero, R; Simal-Gándara, J; Astray, G; Cid, A; Mejuto, JC; Manso, JA; Morales, J. Starch derived cyclodextrins and its future in food biopolymer industry. Starch-Based Polymeric Materials and Nanocomposites: Chemistry, Processing, and Applications; Ahmed, J, Tiwari, B, Imam, SH, Rao, MA, Eds.; CRC Press: Boca Raton, FL, USA, January 2012. Available online: http://www.crcpress.com/product/isbn/9781439851166 (accessed on 4 April 2011).
- Domańska, U; Pobudkowska, A; Pelczarska, A; Winiarska-Tusznio, M; Gierycz, P. Solubility and pKa of select pharmaceutical in water, ethanol, and 1-octanol. J. Chem. Thermodyn 2010, 42, 1465–1472. [Google Scholar]
- Domańska, U; Pobudkowska, A; Pelczarska, A; Gierycz, P. pKa and solubility of drugs in water, ethanol, and 1-octanol. J. Phys. Chem. B 2009, 113, 8941–8947. [Google Scholar]
- Rytting, E; Lentz, KA; Chen, X-Q; Qian, F; Venkatesh, S. Aqueous and cosolvent solubility data for drug-like organic compounds. AAPS J 2005, 7, E78–E105. [Google Scholar]
- Avdeef, A; Bendels, S; Tsinman, O; Tsinman, K; Kansy, M. Solubility-excipient classification gradient maps. Pharm. Res 2007, 42, 530–545. [Google Scholar]
- Avdeef, A. Solubility of sparingly-soluble ionizable drugs. Adv. Drug Deliv. Rev 2007, 59, 568–590. [Google Scholar]
- Pop, MM; Goubitz, K; Borodi, G; Bogdan, M; De Ridder, DJA; Peschar, R; Schenk, H. Crystal structure of the inclusion complex of β-cyclodextrin with mefenamic acid from high-resolution synchrotron powder-diffraction data in combination with molecular-mechanics calculations. Acta Cryst 2002, B58, 1036–1043. [Google Scholar]
- Insel, PA. The Pharmacological Basis of Therapeutics; Goodman Gilman, A, Ed.; McGraw-Hill: New York, NY, USA, 1996; Chapter 27. [Google Scholar]
- El-Kemary, M; El-Shamy, H; Mosaad, MM. The role of capping agent on the interaction of cadmium sulphide nanoparticles with flufenamic acid drug. Mater. Chem. Phys 2009, 118, 81–85. [Google Scholar]
- Sabry, SM. Determination of flufenamic and mefenamic acids in pharmaceutical preparations using organized media. Anal. Chim. Acta 1998, 367, 41–53. [Google Scholar]
- Zielenkiewicz, W; Terekhova, IV; Koźbiał, M; Wszelaka-Rylik, M; Kumeev, RS. Complexation of niflumic acid with native and hydroxypropylated α and β-cyclodextrins in aqueous solution. J. Phys. Org. Chem 2008, 21, 859–866. [Google Scholar]
- Terekhova, IV; Volkova, TV; Perlovich, GL. Experimental analysis of complex formation of niflumic acid with β-Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem 2006, 55, 335–340. [Google Scholar]
- Bogdan, M; Caira, MR; Farcas, SI. Inclusion of the niflumic acid anion in β-cyclodextrin: A solution NMR and X-ray structural investigation. Supramol. Chem 2002, 14, 427–436. [Google Scholar]
- Mura, P; Corti, G; Cirri, M; Maestrelli, F; Mennini, N; Bragagni, M. Development of mucoadhesive films for buccal administration of flufenamic acid: Effect of cyclodextrin complexation. J. Pharm. Sci 2010, 99, 3019–3024. [Google Scholar]
- El Gezawi, S; Omar, N; El Rabbat, N; Ueda, H; Perrin, JH. Microcalorimetric and chromatographic investigations of the binding of some pyridine derivatives to cyclodextrins. J. Pharm. Biomed. Anal 1988, 6, 399–406. [Google Scholar]
- Higuchi, T; Connors, KA. Phase solubility techniques. Adv. Anal. Chem. Instrum 1965, 4, 117–212. [Google Scholar]


| pH | T/K | CD/mM | R | Ks/M−1 | Kd·103/M |
|---|---|---|---|---|---|
| Mefenamic acid | |||||
| 2 | 298.15 | 0.001 | 6.4 | 539 | 1.86 |
| 310.15 | 0.001 | 17.3 | 1627 | 0.62 | |
| 7 | 298.15 | 0.155 | 4.6 | 383 | 2.61 |
| 310.15 | 0.220 | 6.0 | 562 | 1.78 | |
| Niflumic acid | |||||
| 2 | 298.15 | 0.698 | 1.3 | 26 | 38.9 |
| 310.15 | 0.925 | 1.3 | 33 | 29.2 | |
| 7 | 298.15 | 5.754 | 1.6 | 96 | 10.4 |
| 310.15 | 8.171 | 1.5 | 82 | 12.2 | |
| Flufenamic acid | |||||
| 2 | 298.15 | 0.006 | 31.0 | 3055 | 0.33 |
| 310.15 | 0.015 | 19.0 | 1850 | 0.54 | |
| 7 | 298.15 | 0.228 | 2.9 | 202 | 4.95 |
| 310.15 | 0.298 | 3.0 | 218 | 4.59 | |
| Drug | ΔG/(kJ·mol−1) | ΔH/(kJ·mol−1) | ΔS/(J·mol−1·K−1) |
|---|---|---|---|
| MEF | −14.7 | −24.6 | −33.0 |
| NIF | −11.3 | −10.0 | 4.3 |
| FLU | −19.9 | −4.9 | 50.2 |
| Name of compound/abbreviation | Structural formula | M/g · mol−1 |
|---|---|---|
| Mefenamic acid/MEF |  | 241.30 |
| Niflumic acid/NIF |  | 282.22 |
| Flufenamic acid/FLU |  | 281.23 |
| (2-Hydroxypropyl)-β-cyclodextrin |  | average ∼1.460 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Domańska, U.; Pelczarska, A.; Pobudkowska, A. Effect of 2-Hydroxypropyl-β-cyclodextrin on Solubility of Sparingly Soluble Drug Derivatives of Anthranilic Acid. Int. J. Mol. Sci. 2011, 12, 2383-2394. https://doi.org/10.3390/ijms12042383
Domańska U, Pelczarska A, Pobudkowska A. Effect of 2-Hydroxypropyl-β-cyclodextrin on Solubility of Sparingly Soluble Drug Derivatives of Anthranilic Acid. International Journal of Molecular Sciences. 2011; 12(4):2383-2394. https://doi.org/10.3390/ijms12042383
Chicago/Turabian StyleDomańska, Urszula, Aleksandra Pelczarska, and Aneta Pobudkowska. 2011. "Effect of 2-Hydroxypropyl-β-cyclodextrin on Solubility of Sparingly Soluble Drug Derivatives of Anthranilic Acid" International Journal of Molecular Sciences 12, no. 4: 2383-2394. https://doi.org/10.3390/ijms12042383
APA StyleDomańska, U., Pelczarska, A., & Pobudkowska, A. (2011). Effect of 2-Hydroxypropyl-β-cyclodextrin on Solubility of Sparingly Soluble Drug Derivatives of Anthranilic Acid. International Journal of Molecular Sciences, 12(4), 2383-2394. https://doi.org/10.3390/ijms12042383