Immobilization of Ferrocene-Modified SNAP-Fusion Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Ferrocene Modified Benzylguanine-Conjugates


2.2. Ligation to Proteins
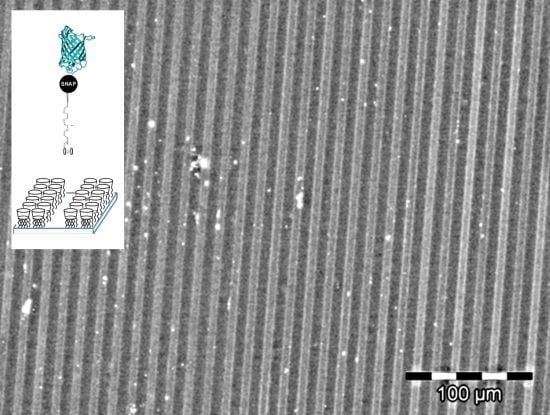
2.3. Immobilization of Proteins


2.4. Immobilization on Vesicles

3. Experimental Section
3.1. Synthesis and Characterization
3.1.1. Monovalent Ferrocene Guest Molecule 3
3.1.2. NHS Ester 5
3.1.3. Bis Alkyne Benzylguanine 7
3.1.4. Bis Ferrocene Benzylguanine 9
3.2. Plasmid Construction, Protein Expression and Purification and Ligation
3.3. Protein Ligation


3.4. Surface Plasmon Resonance
3.5. Preparation of Patterned Host Surfaces by UV-Lithography
3.6. Protein Immobilization on Patterned Surfaces
3.7. Cyclodextrin Vesicles
4. Conclusions
Acknowledgments
References
- Wong, L.S.; Khan, F.; Micklefield, J. Selective covalent protein immobilization: Strategies and applications. Chem. Rev. 2009, 109, 4025–4053. [Google Scholar] [CrossRef]
- Weinrich, D.; Jonkheijm, P.; Niemeyer, C.; Waldmann, H. Applications of protein biochips in biomedical and biotechnological research. Angew. Chem. Int. Ed. 2009, 10, 7744–7751. [Google Scholar]
- Colpo, P.; Ruiz, A.; Ceriotti, L.; Rossi, F. Surface functionalization for protein and cell patterning. Adv. Biochem. Eng. Biotechnol. 2010, 117, 109–130. [Google Scholar]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilization. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef]
- Yang, L.; Gómez-Casado, A.; Young, J.F.; Nguyen, H.D.; Cabanas-Danés, J.; Huskens, J.; Brunsveld, L.; Jonkheijm, P. Reversible and oriented immobilization of ferrocene-modified proteins. J. Am. Chem. Soc. 2012, 134, 19199–19206. [Google Scholar]
- Escalante, M.; Zhao, Y.; Ludden, M.J.W.; Vermeij, R.; Olsen, J.D.; Berenschot, E.; Hunter, C.N.; Huskens, J.; Subramaniam, V.; Otto, C. Nanometer arrays of functional light harvesting antenna complexes by nanoimprint lithography and host-guest interactions. J. Am. Chem. Soc. 2008, 130, 8892–8893. [Google Scholar]
- Hwang, I.; Baek, K.; Jung, M.; Kim, Y.; Park, K.M.; Lee, D.-W.; Selvapalam, N.; Kim, K. Noncovalent immobilization of proteins on a solid surface by cucurbit[7]uril-ferrocenemethylammonium pair, a potential replacement of biotin-avidin pair. J. Am. Chem. Soc. 2007, 129, 4170–4171. [Google Scholar]
- Ludden, M.J.W.; Li, X.; Greve, J.; van Amerongen, A.; Escalante, M.; Subramaniam, V.; Reinhoudt, D.N.; Huskens, J. Assembly of bionanostructures onto beta-cyclodextrin molecular printboards for antibody recognition and lymphocyte cell counting. J. Am. Chem. Soc. 2008, 130, 6964–6973. [Google Scholar]
- Ludden, M.L.W.; Mulder, A.; Schulze, K.; Subramaniam, V.; Tampé, R.; Huskens, J. Anchoring of histidine-tagged proteins to molecular printboards: Self-assembly, thermodynamic modeling, and patterning. Chem. Eur. J. 2008, 14, 2044–2051. [Google Scholar]
- González-Campo, A.; Brasch, M.; Uhlenheuer, D.; Gómez-Casado, A.; Yang, L.; Brunsveld, L.; Huskens, J.; Jonkheijm, P. Supramolecularly oriented immobilization of proteins using cucurbit[8]uril. Langmuir 2012. [Google Scholar] [CrossRef]
- González-Campo, A.; Eker, B.; Gardeniers, H.J.G.E.; Huskens, J.; Jonkheijm, P. A supramolecular approach to enzyme immobilization in micro-channels. Small 2012, 8, 3531–3537. [Google Scholar] [CrossRef]
- Felici, M.; Marzá-Pérez, M.; Hatzakis, N.S.; Nolte, R.J.M.; Feiters, M.C. Beta-Cyclodextrin-appended giant amphiphile: Aggregation to vesicle polymersomes and immobilisation of enzymes. Chem. Eur. J. 2008, 14, 9914–9920. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Brunsveld, L. A synthetic supramolecular construct modulating protein assembly in cells. Angew. Chem. Int. Ed. 2007, 46, 1798–1802. [Google Scholar] [CrossRef]
- Uhlenheuer, D.A.; Wasserberg, D.; Nguyen, H.; Zhang, L.; Blum, C.; Subramaniam, V.; Brunsveld, L. Modulation of protein dimerization by a supramolecular host-guest system. Chem. Eur. J. 2009, 15, 8779–8790. [Google Scholar]
- Uhlenheuer, D.A.; Young, J.F.; Nguyen, H.D.; Scheepstra, M.; Brunsveld, L. Cucurbit[8]uril induced heterodimerization of methylviologen and naphthalene functionalized proteins. Chem. Commun. 2011, 47, 6798–6800. [Google Scholar]
- Uhlenheuer, D.; Milroy, L.-G.; Neirynck, P.; Brunsveld, L. Strong supramolecular control over protein self-assembly using a polyamine decorated beta-cyclodextrin as synthetic recognition element. J. Mater. Chem. 2011, 21, 18919–18922. [Google Scholar] [CrossRef]
- Young, J.F.; Nguyen, H.D.; Yang, L.; Huskens, J.; Jonkheijm, P.; Brunsveld, L. Strong and reversible monovalent supramolecular protein immobilization. ChemBioChem 2010, 1, 180–183. [Google Scholar]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechn. 2002, 21, 86–89. [Google Scholar]
- Juillerat, A.; Gronemeyer, T.; Keppler, A.; Gendreizig, S.; Pick, H.; Vogel, H.; Johnsson, K. Directed evolution of O6-alkylguanine-DNA alkyltransferase for efficient labeling of fusion proteins with small molecules in vivo. Chem. Biol. 2003, 10, 313–317. [Google Scholar] [CrossRef]
- Hinner, M.J.; Johnsson, K. How to obtain labeled proteins and what to do with them. Curr. Opin. Biotechnol. 2010, 21, 766–776. [Google Scholar] [CrossRef]
- Kindermann, M.; George, N.; Johnsson, N.; Johnsson, K. Covalent and selective immobilization of fusion proteins. J. Am. Chem. Soc. 2003, 125, 7810–7811. [Google Scholar] [CrossRef]
- Sielaff, I.; Arnold, A.; Godin, G.; Tugulu, S.; Klok, H.A.; Johnsson, K. Protein function microarrays based on self-immobilizing and self-labeling fusion proteins. ChemBioChem 2006, 7, 194–202. [Google Scholar] [CrossRef]
- Iversen, L.; Cherouati, N.; Berthing, T.; Stamou, D.; Martinez, K.L. Templated protein assembly on micro-contact-printed surface patterns. Use of the SNAP-tag protein functionality. Langmuir 2008, 24, 6375–6381. [Google Scholar] [CrossRef]
- Recker, T.; Haamann, D.; Schmitt, A.; Kuster, A.; Klee, D.; Barth, S.; Muller-Newen, G. Directed covalent immobilization of fluorescently labeled cytokines. Bioconj. Chem. 2011, 22, 1210–1220. [Google Scholar] [CrossRef]
- Engin, S.; Trouillet, V.; Franz, C.M.; Welle, A.; Bruns, M.; Wedlich, D. Benzylguanine thiol self-assembled monolayers for the immobilization of SNAP-tag proteins on microcontact-printed surface structures. Langmuir 2010, 26, 6097–6101. [Google Scholar]
- Uhlenheuer, D.A.; Wasserberg, D.; Haase, C.; Nguyen, H.D.; Schenkel, J.H.; Huskens, J.; Ravoo, B.J.; Jonkheijm, P.; Brunsveld, L. Directed supramolecular surface assembly of SNAP-tag fusion proteins. Chem. Eur. J. 2012, 18, 6788–6794. [Google Scholar]
- Falvey, P.; Lim, C.W.; Darcy, R.; Revermann, T.; Karst, U.; Giesbers, M.; Marcelis, A.T.M.; Lazar, A.; Coleman, A.W.; Reinhoudt, D.N.; et al. Bilayer vesicles of amphiphilic cyclodextrins: Host membranes that recognize guest molecules. Chem. Eur. J. 2005, 11, 1171–1180. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Lemercier, G.; Gendreizig, S.; Kindermann, M.; Johnsson, K. Inducing and sensing protein–protein interactions in living cells by selective cross-linking. Angew. Chem. Int. Ed. 2007, 46, 4281–4284. [Google Scholar] [CrossRef]
- Uhlenheuer, D.A. Supramolecular control over protein assembly. PhD Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 19 September 2011. [Google Scholar]
- Rijkers, D.T.; van Esse, G.W.; Merkx, R.; Brouwer, A.J.; Jacobs, H.J.; Pieters, R.J.; Liskamp, R.M. Efficient microwave-assisted synthesis of multivalent dendrimeric peptides using cycloaddition reaction (click) chemistry. Chem. Commun. 2005, 4581–4583. [Google Scholar]
- Kobayashi, T.; Komatsu, T.; Terai, T.; Hanaoka, K.; Nagano, T.; Urano, Y.; Kamiya, M.; Campos, C.; Gonzalez-Gaitan, M. Highly activatable and environment-insensitive optical highlighters for selective spatiotemporal imaging of target proteins. J. Am. Chem. Soc. 2012, 134, 11153–11160. [Google Scholar]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Onclin, S.; Mulder, A.; Huskens, J.; Ravoo, B.J.; Reinhoudt, D.N. Molecular printboards: monolayers of beta-cyclodextrins on silicon oxide surfaces. Langmuir 2004, 20, 5460–5466. [Google Scholar] [CrossRef]
- Beulen, M.W.; Bügler, J.; de Jong, M.R.; Lammerink, B.; Huskens, J.; Schönherr, H.; Vancso, G.J.; Boukamp, B.A.; Wieder, H.; Offenhäuser, A.; et al. Host-guest interactions at self-assembled monolayers of cyclodextrins on gold. Chem. Eur. J. 2000, 6, 1176–1183. [Google Scholar]
- Gomez-Casado, A.; Jonkheijm, P.; Huskens, J. Recognition properties of cucurbit[7]uril self-assembled monolayers studied with force spectroscopy. Langmuir 2011, 27, 11508–11513. [Google Scholar] [CrossRef]
- Alker, D.; Ashton, P.R.; Harding, V.D.; Königer, R.; Stoddart, J.F. Amino acid derivatives of beta-cyclodextrin. J. Org. Chem. 1996, 61, 903–908. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wasserberg, D.; Uhlenheuer, D.A.; Neirynck, P.; Cabanas-Danés, J.; Schenkel, J.H.; Ravoo, B.J.; An, Q.; Huskens, J.; Milroy, L.-G.; Brunsveld, L.; et al. Immobilization of Ferrocene-Modified SNAP-Fusion Proteins. Int. J. Mol. Sci. 2013, 14, 4066-4080. https://doi.org/10.3390/ijms14024066
Wasserberg D, Uhlenheuer DA, Neirynck P, Cabanas-Danés J, Schenkel JH, Ravoo BJ, An Q, Huskens J, Milroy L-G, Brunsveld L, et al. Immobilization of Ferrocene-Modified SNAP-Fusion Proteins. International Journal of Molecular Sciences. 2013; 14(2):4066-4080. https://doi.org/10.3390/ijms14024066
Chicago/Turabian StyleWasserberg, Dorothee, Dana A. Uhlenheuer, Pauline Neirynck, Jordi Cabanas-Danés, Jan Hendrik Schenkel, Bart Jan Ravoo, Qi An, Jurriaan Huskens, Lech-Gustav Milroy, Luc Brunsveld, and et al. 2013. "Immobilization of Ferrocene-Modified SNAP-Fusion Proteins" International Journal of Molecular Sciences 14, no. 2: 4066-4080. https://doi.org/10.3390/ijms14024066
APA StyleWasserberg, D., Uhlenheuer, D. A., Neirynck, P., Cabanas-Danés, J., Schenkel, J. H., Ravoo, B. J., An, Q., Huskens, J., Milroy, L. -G., Brunsveld, L., & Jonkheijm, P. (2013). Immobilization of Ferrocene-Modified SNAP-Fusion Proteins. International Journal of Molecular Sciences, 14(2), 4066-4080. https://doi.org/10.3390/ijms14024066