Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia
Abstract
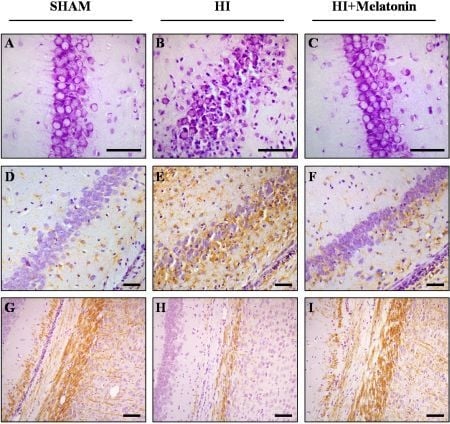
:1. Introduction
2. Brain Protection
3. Antioxidant
4. Anti-Apoptotic
5. Anti-Inflammatory
6. Conclusions
Conflict of Interest
References
- Arendt, J. Melatonin and the Mammalian Pineal Gland; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res 2007, 42, 28–42. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc 2010, 85, 607–623. [Google Scholar]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res 2006, 41, 374–381. [Google Scholar]
- Acuna-Castroviejo, D.; Martin, M.; Macias, M.; Escames, G.; Leon, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res 2001, 30, 65–74. [Google Scholar]
- Tomas-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res 2005, 39, 99–104. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. A Review. J. Biomed. Sci 2000, 7, 444–458. [Google Scholar]
- De Haan, M.; Wyatt, J.S.; Roth, S.; Vargha-Khadem, F.; Gadian, D.; Mishkin, M. Brain and cognitive-behavioural development after asphyxia at term birth. Dev. Sci 2006, 9, 350–358. [Google Scholar]
- Du Plessis, A.J.; Volpe, J.J. Perinatal brain injury in the preterm and term newborn. Curr. Opin. Neurol 2002, 15, 151–157. [Google Scholar]
- Hamrick, S.E.; Ferriero, D.M. The injury response in the term newborn brain: Can we neuroprotect? Curr. Opin. Neurol 2003, 16, 147–154. [Google Scholar]
- Volpe, J.J. Perinatal brain injury: From pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev 2001, 7, 56–64. [Google Scholar]
- Low, J.A. Determining the contribution of asphyxia to brain damage in the neonate. J. Obstet. Gynaecol. Res 2004, 30, 276–286. [Google Scholar]
- Vannucci, S.J.; Hagberg, H. Hypoxia-ischemia in the immature brain. J. Exp. Biol 2004, 207, 3149–3154. [Google Scholar]
- Maneru, C.; Junque, C.; Botet, F.; Tallada, M.; Guardia, J. Neuropsychological long-term sequelae of perinatal asphyxia. Brain Inj 2001, 15, 1029–1039. [Google Scholar]
- Yager, J.Y.; Armstrong, E.A.; Black, A.M. Treatment of the term newborn with brain injury: Simplicity as the mother of invention. Pediatr. Neurol 2009, 40, 237–243. [Google Scholar]
- Vitte, P.A.; Harthe, C.; Lestage, P.; Claustrat, B.; Bobillier, P. Plasma, cerebrospinal fluid, and brain distribution of 14C-melatonin in Rat: A biochemical and autoradiographic study. J. Pineal Res 1988, 5, 437–453. [Google Scholar]
- Menendez-Pelaez, A.; Reiter, R.J. Distribution of melatonin in mammalian tissues: The relative importance of nuclear versus cytosolic localization. J. Pineal Res 1993, 15, 59–69. [Google Scholar]
- Gupta, Y.K.; Gupta, M.; Kohli, K. Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian J. Physiol. Pharmacol 2003, 47, 373–386. [Google Scholar]
- Rees, S.; Harding, R.; Walker, D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int. J. Dev. Neurosci 2011, 29, 551–563. [Google Scholar]
- Hilario, E.; Alvarez, A.; Alvarez, F.J.; Gastiasoro, E.; Valls-i-Soler, A. Cellular mechanisms in perinatal hypoxic-ischemic brain injury. Curr. Pediatr. Rev 2006, 2, 131–141. [Google Scholar]
- Manev, H.; Uz, T.; Kharlamov, A.; Joo, J.Y. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. FASEB J 1996, 10, 1546–1551. [Google Scholar]
- Li, X.J.; Zhang, L.M.; Gu, J.; Zhang, A.Z.; Sun, F.Y. Melatonin decreases production of hydroxyl radical during cerebral ischemia-reperfusion. Zhongguo Yao Li Xue Bao 1997, 18, 394–396. [Google Scholar]
- Cho, S.; Joh, T.H.; Baik, H.H.; Dibinis, C.; Volpe, B.T. Melatonin administration protects CA1 hippocampal neurons after transient forebrain ischemia in rats. Brain Res 1997, 755, 335–338. [Google Scholar]
- Kilic, E.; Ozdemir, Y.G.; Bolay, H.; Kelestimur, H.; Dalkara, T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow MeTable 1999, 19, 511–516. [Google Scholar]
- Wakatsuki, A.; Okatani, Y.; Izumiya, C.; Ikenoue, N. Melatonin protects against ischemia and reperfusion-induced oxidative lipid and DNA damage in fetal rat brain. J. Pineal Res 1999, 26, 147–152. [Google Scholar]
- Joo, J.Y.; Uz, T.; Manev, H. Opposite effects of pinealectomy and melatonin administration on brain damage following cerebral focal ischemia in rat. Restor. Neurol. Neurosci 1998, 13, 185–191. [Google Scholar]
- Cuzzocrea, S.; Costantino, G.; Gitto, E.; Mazzon, E.; Fulia, F.; Serraino, I.; Cordaro, S.; Barberi, I.; De Sarro, A.; Caputi, A.P. Protective effects of melatonin in ischemic brain injury. J. Pineal Res 2000, 29, 217–227. [Google Scholar]
- Letechipia-Vallejo, G.; Gonzalez-Burgos, I.; Cervantes, M. Neuroprotective effect of melatonin on brain damage induced by acute global cerebral ischemia in cats. Arch. Med. Res 2001, 32, 186–192. [Google Scholar]
- Zhang, J.; Guo, J.D.; Xing, S.H.; Gu, S.L.; Dai, T.J. The protective effects of melatonin on global cerebral ischemia-reperfusion injury in gerbils. Yao Xue Xue Bao 2002, 37, 329–333. [Google Scholar]
- Pei, Z.; Pang, S.F.; Cheung, R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 2003, 34, 770–775. [Google Scholar]
- Koh, P.O. Melatonin attenuates the focal cerebral ischemic injury by inhibiting the dissociation of pBad from 14-3-3. J. Pineal Res 2008, 44, 101–106. [Google Scholar]
- Wang, X.; Figueroa, B.E.; Stavrovskaya, I.G.; Zhang, Y.; Sirianni, A.C.; Zhu, S.; Day, A.L.; Kristal, B.S.; Friedlander, R.M. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke 2009, 40, 1877–1885. [Google Scholar]
- Alonso-Alconada, D.; Alvarez, A.; Lacalle, J.; Hilario, E. Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia-ischemia. Histol. Histopathol 2012, 27, 771–783. [Google Scholar]
- Carloni, S.; Perrone, S.; Buonocore, G.; Longini, M.; Proietti, F.; Balduini, W. Melatonin protects from the long-term consequences of a neonatal hypoxic-ischemic brain injury in rats. J. Pineal Res 2008, 44, 157–164. [Google Scholar]
- Cetinkaya, M.; Alkan, T.; Ozyener, F.; Kafa, I.M.; Kurt, M.A.; Koksal, N. Possible neuroprotective effects of magnesium sulfate and melatonin as both pre- and post-treatment in a neonatal hypoxic-ischemic rat model. Neonatology 2011, 99, 302–310. [Google Scholar]
- Ozyener, F.; Cetinkaya, M.; Alkan, T.; Goren, B.; Kafa, I.M.; Kurt, M.A.; Koksal, N. Neuroprotective effects of melatonin administered alone or in combination with topiramate in neonatal hypoxic-ischemic rat model. Restor. Neurol. Neurosci 2012, 30, 435–444. [Google Scholar]
- Mattson, M.P.; Guthrie, P.B.; Kater, S.B. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog. Clin. Biol. Res 1989, 317, 333–351. [Google Scholar]
- Johnston, M.V. Selective vulnerability in the neonatal brain. Ann. Neurol 1998, 44, 155–156. [Google Scholar]
- Northington, F.J.; Ferriero, D.M.; Graham, E.M.; Traystman, R.J.; Martin, L.J. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol. Dis 2001, 8, 207–219. [Google Scholar]
- Hamada, F.; Watanabe, K.; Wakatsuki, A.; Nagai, R.; Shinohara, K.; Hayashi, Y.; Imamura, R.; Fukaya, T. Therapeutic effects of maternal melatonin administration on ischemia/reperfusion-induced oxidative cerebral damage in neonatal rats. Neonatology 2010, 98, 33–40. [Google Scholar]
- Watanabe, K.; Hamada, F.; Wakatsuki, A.; Nagai, R.; Shinohara, K.; Hayashi, Y.; Imamura, R.; Fukaya, T. Prophylactic administration of melatonin to the mother throughout pregnancy can protect against oxidative cerebral damage in neonatal rats. J. Matern. Fetal. Neonatal Med 2012, 25, 1254–1259. [Google Scholar]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol 2004, 72, 111–127. [Google Scholar]
- Panickar, K.S.; Norenberg, M.D. Astrocytes in cerebral ischemic injury: Morphological and general considerations. Glia 2005, 50, 287–298. [Google Scholar]
- Sizonenko, S.V.; Camm, E.J.; Dayer, A.; Kiss, J.Z. Glial responses to neonatal hypoxic-ischemic injury in the rat cerebral cortex. Int. J. Dev. Neurosci 2008, 26, 37–45. [Google Scholar]
- Huang, Z.; Liu, J.; Cheung, P.Y.; Chen, C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res 2009, 1301, 100–109. [Google Scholar]
- Xiong, M.; Yang, Y.; Chen, G.Q.; Zhou, W.H. Post-ischemic hypothermia for 24h in P7 rats rescues hippocampal neuron: Association with decreased astrocyte activation and inflammatory cytokine expression. Brain Res. Bull 2009, 79, 351–357. [Google Scholar]
- Rothstein, R.P.; Levison, S.W. Gray matter oligodendrocyte progenitors and neurons die caspase-3 mediated deaths subsequent to mild perinatal hypoxic/ischemic insults. Dev. Neurosci 2005, 27, 149–159. [Google Scholar]
- Inder, T.E.; Wells, S.J.; Mogridge, N.B.; Spencer, C.; Volpe, J.J. Defining the nature of the cerebral abnormalities in the premature infant: A qualitative magnetic resonance imaging study. J. Pediatr 2003, 143, 171–179. [Google Scholar]
- Wang, X.; Hagberg, H.; Zhu, C.; Jacobsson, B.; Mallard, C. Effects of intrauterine inflammation on the developing mouse brain. Brain Res 2007, 1144, 180–185. [Google Scholar]
- Olivier, P.; Fontaine, R.H.; Loron, G.; van Steenwinckel, J.; Biran, V.; Massonneau, V.; Kaindl, A.; Dalous, J.; Charriaut-Marlangue, C.; Aigrot, M.S.; et al. Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS One 2009, 4, e7128. [Google Scholar]
- Kaur, C.; Sivakumar, V.; Ling, E.A. Melatonin protects periventricular white matter from damage due to hypoxia. J. Pineal Res 2010, 48, 185–193. [Google Scholar]
- Villapol, S.; Fau, S.; Renolleau, S.; Biran, V.; Charriaut-Marlangue, C.; Baud, O. Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr. Res 2011, 69, 51–55. [Google Scholar]
- McLean, C.; Ferriero, D. Mechanisms of hypoxic-ischemic injury in the term infant. Semin. Perinatol 2004, 28, 425–432. [Google Scholar]
- Sheldon, R.A.; Jiang, X.; Francisco, C.; Christen, S.; Vexler, Z.S.; Tauber, M.G.; Ferriero, D.M. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr. Res 2004, 56, 656–662. [Google Scholar]
- McQuillen, P.S.; Ferriero, D.M. Selective vulnerability in the developing central nervous system. Pediatr. Neurol 2004, 30, 227–235. [Google Scholar]
- Miller, S.L.; Yan, E.B.; Castillo-Melendez, M.; Jenkin, G.; Walker, D.W. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev. Neurosci 2005, 27, 200–210. [Google Scholar]
- Tutunculer, F.; Eskiocak, S.; Basaran, U.N.; Ekuklu, G.; Ayvaz, S.; Vatansever, U. The protective role of melatonin in experimental hypoxic brain damage. Pediatr. Int 2005, 47, 434–439. [Google Scholar]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res 2001, 31, 343–349. [Google Scholar]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol 2002, 30, 620–650. [Google Scholar]
- Arneson, K.O.; Roberts, L.J., 2nd. Measurement of products of docosahexaenoic acid peroxidation, neuroprostanes, and neurofurans. Methods Enzymol 2007, 433, 127–143. [Google Scholar]
- Song, W.L.; Lawson, J.A.; Reilly, D.; Rokach, J.; Chang, C.T.; Giasson, B.; FitzGerald, G.A. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J. Biol. Chem 2008, 283, 6–16. [Google Scholar]
- Signorini, C.; Ciccoli, L.; Leoncini, S.; Carloni, S.; Perrone, S.; Comporti, M.; Balduini, W.; Buonocore, G. Free iron, total F-isoprostanes and total F-neuroprostanes in a model of neonatal hypoxic-ischemic encephalopathy: Neuroprotective effect of melatonin. J. Pineal Res 2009, 46, 148–154. [Google Scholar]
- Balduini, W.; Carloni, S.; Perrone, S.; Bertrando, S.; Tataranno, M.L.; Negro, S.; Proietti, F.; Longini, M.; Buonocore, G. The use of melatonin in hypoxic-ischemic brain damage: An experimental study. J. Matern. Fetal. Neonatal Med 2012, 25, 119–124. [Google Scholar]
- Welin, A.K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr. Res 2007, 61, 153–158. [Google Scholar]
- Eskiocak, S.; Tutunculer, F.; Basaran, U.N.; Taskiran, A.; Cakir, E. The Effect of melatonin on protein oxidation and nitric oxide in the brain tissue of hypoxic neonatal rats. Brain Dev 2007, 29, 19–24. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996, 49, 1304–1313. [Google Scholar]
- Andrabi, S.A.; Sayeed, I.; Siemen, D.; Wolf, G.; Horn, T.F. Direct Inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J 2004, 18, 869–871. [Google Scholar]
- Wang, X.; Zhu, S.; Pei, Z.; Drozda, M.; Stavrovskaya, I.G.; Del Signore, S.J.; Cormier, K.; Shimony, E.M.; Wang, H.; Ferrante, R.J.; et al. Inhibitors of cytochrome c release with therapeutic potential for huntington’s disease. J. Neurosci 2008, 28, 9473–9485. [Google Scholar]
- Jou, M.J.; Peng, T.I.; Reiter, R.J.; Jou, S.B.; Wu, H.Y.; Wen, S.T. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res 2004, 37, 55–70. [Google Scholar]
- Kilic, E.; Kilic, U.; Reiter, R.J.; Bassetti, C.L.; Hermann, D.M. Tissue-plasminogen activator-induced ischemic brain injury is reversed by melatonin: Role of inos and akt. J. Pineal Res 2005, 39, 151–155. [Google Scholar]
- Jang, M.H.; Jung, S.B.; Lee, M.H.; Kim, C.J.; Oh, Y.T.; Kang, I.; Kim, J.; Kim, E.H. Melatonin attenuates amyloid beta25–35-induced apoptosis in mouse microglial bv2 cells. Neurosci. Lett 2005, 380, 26–31. [Google Scholar]
- Ebadi, M.; Sharma, S.K.; Ghafourifar, P.; Brown-Borg, H.; El Refaey, H. Peroxynitrite in the pathogenesis of parkinson’s disease and the neuroprotective role of metallothioneins. Methods Enzymol 2005, 396, 276–298. [Google Scholar]
- Alvira, D.; Tajes, M.; Verdaguer, E.; Acuna-Castroviejo, D.; Folch, J.; Camins, A.; Pallas, M. Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of parkinson’s disease. J. Pineal Res 2006, 40, 251–258. [Google Scholar]
- Ling, X.; Zhang, L.M.; Lu, S.D.; Li, X.J.; Sun, F.Y. Protective effect of melatonin on injuried cerebral neurons is associated with bcl-2 protein over-expression. Zhongguo Yao Li Xue Bao 1999, 20, 409–414. [Google Scholar]
- Sun, F.Y.; Lin, X.; Mao, L.Z.; Ge, W.H.; Zhang, L.M.; Huang, Y.L.; Gu, J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res 2002, 33, 48–56. [Google Scholar]
- Chung, S.Y.; Han, S.H. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J. Pineal Res 2003, 34, 95–102. [Google Scholar]
- Feng, Z.; Cheng, Y.; Zhang, J.T. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J. Pineal Res 2004, 37, 198–206. [Google Scholar]
- Deng, Y.Q.; Xu, G.G.; Duan, P.; Zhang, Q.; Wang, J.Z. Effects of melatonin on wortmannin-induced TAU hyperphosphorylation. Acta Pharmacol. Sin 2005, 26, 519–526. [Google Scholar]
- Rosenstein, R.E.; Cardinali, D.P. Central gabaergic mechanisms as targets for melatonin activity in brain. Neurochem. Int 1990, 17, 373–379. [Google Scholar]
- Molina-Carballo, A.; Munoz-Hoyos, A.; Sanchez-Forte, M.; Uberos-Fernandez, J.; Moreno-Madrid, F.; Acuna-Castroviejo, D. Melatonin increases following convulsive seizures may be related to its anticonvulsant properties at physiological concentrations. Neuropediatrics 2007, 38, 122–125. [Google Scholar]
- Prada, C.; Udin, S.B.; Wiechmann, A.F.; Zhdanova, I.V. Stimulation of melatonin receptors decreases calcium levels in xenopus tectal cells by activating gaba(c) receptors. J. Neurophysiol 2005, 94, 968–978. [Google Scholar]
- Prada, C.; Udin, S.B. Melatonin decreases calcium levels in retinotectal axons of xenopus laevis by indirect activation of group iii metabotropic glutamate receptors. Brain Res 2005, 1053, 67–76. [Google Scholar]
- Buonocore, G.; Perrone, S.; Bracci, R. Free radicals and brain damage in the newborn. Biol. Neonate 2001, 79, 180–186. [Google Scholar]
- Blomgren, K.; Hagberg, H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic. Biol. Med 2006, 40, 388–397. [Google Scholar]
- Kumar, A.; Mittal, R.; Khanna, H.D.; Basu, S. Free radical injury and blood-brain barrier permeability in hypoxic-ischemic encephalopathy. Pediatrics 2008, 122, 722–727. [Google Scholar]
- Alonso-Alconada, D.; Hilario, E.; Alvarez, F.J.; Alvarez, A. Apoptotic cell death correlates with ros overproduction and early cytokine expression after hypoxia-ischemia in fetal lambs. Reprod. Sci 2012, 19, 754–763. [Google Scholar]
- Watanabe, K.; Wakatsuki, A.; Shinohara, K.; Ikenoue, N.; Yokota, K.; Fukaya, T. Maternally administered melatonin protects against ischemia and reperfusion-induced oxidative mitochondrial damage in premature fetal rat brain. J. Pineal Res 2004, 37, 276–280. [Google Scholar]
- Hutton, L.C.; Abbass, M.; Dickinson, H.; Ireland, Z.; Walker, D.W. Neuroprotective properties of melatonin in a model of birth asphyxia in the spiny mouse (Acomys cahirinus). Dev. Neurosci 2009, 31, 437–451. [Google Scholar]
- Fu, J.; Zhao, S.D.; Liu, H.J.; Yuan, Q.H.; Liu, S.M.; Zhang, Y.M.; Ling, E.A.; Hao, A.J. Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J. Pineal Res 2011, 51, 104–112. [Google Scholar]
- Kilic, U.; Kilic, E.; Reiter, R.J.; Bassetti, C.L.; Hermann, D.M. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J. Pineal Res 2005, 38, 67–71. [Google Scholar]
- Koh, P.O. Melatonin prevents the injury-induced decline of akt/forkhead transcription factors phosphorylation. J. Pineal Res 2008, 45, 199–203. [Google Scholar]
- Zhou, J.; Zhang, S.; Zhao, X.; Wei, T. Melatonin impairs nadph oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1–42. J. Pineal Res 2008, 45, 157–165. [Google Scholar]
- Koh, P.O. Melatonin prevents ischemic brain injury through activation of the mtor/p70s6 kinase signaling pathway. Neurosci. Lett 2008, 444, 74–78. [Google Scholar]
- Fjaerli, O.; Lund, T.; Osterud, B. The effect of melatonin on cellular activation processes in human blood. J. Pineal Res 1999, 26, 50–55. [Google Scholar]
- Baykal, A.; Iskit, A.B.; Hamaloglu, E.; Guc, M.O.; Hascelik, G.; Sayek, I. Melatonin modulates mesenteric blood flow and TNFα concentrations after lipopolysaccharide challenge. Eur. J. Surg 2000, 166, 722–727. [Google Scholar]
- Silva, S.O.; Rodrigues, M.R.; Ximenes, V.F.; Bueno-da-Silva, A.E.; Amarante-Mendes, G.P.; Campa, A. Neutrophils as a specific target for melatonin and kynuramines: Effects on cytokine release. J. Neuroimmunol 2004, 156, 146–152. [Google Scholar]
- Wang, H.; Wei, W.; Shen, Y.X.; Dong, C.; Zhang, L.L.; Wang, N.P.; Yue, L.; Xu, S.Y. Protective effect of melatonin against liver injury in mice induced by bacillus calmette-guerin plus lipopolysaccharide. World J. Gastroenterol 2004, 10, 2690–2696. [Google Scholar]
- Perianayagam, M.C.; Oxenkrug, G.F.; Jaber, B.L. Immune-modulating effects of melatonin, N-acetylserotonin, and N-acetyldopamine. Ann. N.Y. Acad. Sci 2005, 1053, 386–393. [Google Scholar]
- Carrillo-Vico, A.; Lardone, P.J.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Calvo, J.R.; Karasek, M.; Guerrero, J.M. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. MeTable 2005, 90, 992–1000. [Google Scholar]
- Gitto, E.; Reiter, R.J.; Sabatino, G.; Buonocore, G.; Romeo, C.; Gitto, P.; Bugge, C.; Trimarchi, G.; Barberi, I. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: Improvement with melatonin treatment. J. Pineal Res 2005, 39, 287–293. [Google Scholar]
- Steinhilber, D.; Brungs, M.; Werz, O.; Wiesenberg, I.; Danielsson, C.; Kahlen, J.P.; Nayeri, S.; Schrader, M.; Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem 1995, 270, 7037–7040. [Google Scholar]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5- methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol 2005, 165, 139–149. [Google Scholar]
- Deng, W.G.; Tang, S.T.; Tseng, H.P.; Wu, K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006, 108, 518–524. [Google Scholar]
- Cardinali, D.P.; Ritta, M.N.; Fuentes, A.M.; Gimeno, M.F.; Gimeno, A.L. Prostaglandin E release by rat medial basal hypothalamus in vitro. Inhibition by melatonin at submicromolar concentrations. Eur. J. Pharmacol 1980, 67, 151–153. [Google Scholar]
- Cardinali, D.P.; Ritta, M.N. The role of prostaglandins in neuroendocrine junctions: Studies in the pineal gland and the hypothalamus. Neuroendocrinology 1983, 36, 152–160. [Google Scholar]
- Carrillo-Vico, A.; Garcia-Maurino, S.; Calvo, J.R.; Guerrero, J.M. Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes via its mt1 membrane receptor. FASEB J 2003, 17, 755–757. [Google Scholar]
- Bilici, D.; Akpinar, E.; Kiziltunc, A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol. Res 2002, 46, 133–139. [Google Scholar]
- Acuna-Castroviejo, D.; Escames, G.; Lopez, L.C.; Hitos, A.B.; Leon, J. Melatonin and nitric oxide: Two required antagonists for mitochondrial homeostasis. Endocrine 2005, 27, 159–168. [Google Scholar]
- Leon, J.; Macias, M.; Escames, G.; Camacho, E.; Khaldy, H.; Martin, M.; Espinosa, A.; Gallo, M.A.; Acuna-Castroviejo, D. Structure-related inhibition of calmodulin-dependent neuronal nitric-oxide synthase activity by melatonin and synthetic kynurenines. Mol. Pharmacol 2000, 58, 967–975. [Google Scholar]
- Leon, J.; Escames, G.; Rodriguez, M.I.; Lopez, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrion, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem 2006, 98, 2023–2033. [Google Scholar]
- Jimenez-Ortega, V.; Cano, P.; Cardinali, D.P.; Esquifino, A.I. 24-Hour variation in gene expression of redox pathway enzymes in rat hypothalamus: Effect of melatonin treatment. Redox Rep 2009, 14, 132–138. [Google Scholar]
- Tapias, V.; Escames, G.; Lopez, L.C.; Lopez, A.; Camacho, E.; Carrion, M.D.; Entrena, A.; Gallo, M.A.; Espinosa, A.; Acuna-Castroviejo, D. Melatonin and its brain metabolite N(1)-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J. Neurosci. Res 2009, 87, 3002–3010. [Google Scholar]
- Leon, J.; Vives, F.; Crespo, E.; Camacho, E.; Espinosa, A.; Gallo, M.A.; Escames, G.; Acuna-Castroviejo, D. Modification of nitric oxide synthase activity and neuronal response in rat striatum by melatonin and kynurenine derivatives. J. Neuroendocrinol 1998, 10, 297–302. [Google Scholar]
- Chandrasekaran, A.; Ponnambalam, G.; Kaur, C. Domoic acid-induced neurotoxicity in the hippocampus of adult rats. Neurotox Res 2004, 6, 105–117. [Google Scholar]
- Escames, G.; Khaldy, H.; Leon, J.; Gonzalez, L.; Acuna-Castroviejo, D. Changes in iNOS activity, oxidative stress and melatonin levels in hypertensive patients treated with lacidipine. J. Hypertens 2004, 22, 629–635. [Google Scholar]
- Escames, G.; Acuna-Castroviejo, D.; Lopez, L.C.; Tan, D.X.; Maldonado, M.D.; Sanchez-Hidalgo, M.; Leon, J.; Reiter, R.J. Pharmacological utility of melatonin in the treatment of septic shock: Experimental and clinical evidence. J. Pharm. Pharmacol 2006, 58, 1153–1165. [Google Scholar]
- Escames, G.; Lopez, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; Leon, J.; Rodriguez, M.I.; Acuna-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res 2006, 40, 71–78. [Google Scholar]
- Lopez, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; Leon, J.; Acuna-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol 2006, 38, 267–278. [Google Scholar]
- Srinivasan, V.; Pandi-Perumal, S.R.; Spence, D.W.; Kato, H.; Cardinali, D.P. Melatonin in septic shock: Some recent concepts. J. Crit. Care 2010, 25, 656, e1–656.e6.. [Google Scholar]
- Lopez, L.C.; Escames, G.; Ortiz, F.; Ros, E.; Acuna-Castroviejo, D. Melatonin restores the mitochondrial production of ATP in septic mice. Neuro Endocrinol. Lett 2006, 27, 623–630. [Google Scholar]
- Escames, G.; Lopez, L.C.; Ortiz, F.; Lopez, A.; Garcia, J.A.; Ros, E.; Acuna-Castroviejo, D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 2007, 274, 2135–2147. [Google Scholar]
- Pei, Z.; Cheung, R.T. Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J. Pineal Res 2004, 37, 85–91. [Google Scholar]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.Y.; Chen, S.T.; Huang, C.C.; Yang, I.P.; Hsu, Y.S.; Wu, T.S.; Lee, E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res 2007, 42, 297–309. [Google Scholar]
- Koh, P.O. Melatonin regulates nitric oxide synthase expression in ischemic brain injury. J. Vet. Med. Sci 2008, 70, 747–750. [Google Scholar]
- Mohan, N.; Sadeghi, K.; Reiter, R.J.; Meltz, M.L. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-κB. Biochem. Mol. Biol. Int 1995, 37, 1063–1070. [Google Scholar]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and its relation to the immune system and inflammation. Ann. N.Y. Acad. Sci 2000, 917, 376–386. [Google Scholar]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar]
- Kaur, C.; Sivakumar, V.; Lu, J.; Tang, F.R.; Ling, E.A. Melatonin attenuates hypoxia-induced ultrastructural changes and increased vascular permeability in the developing hippocampus. Brain Pathol 2008, 18, 533–547. [Google Scholar]
- Jan, J.E.; Wasdell, M.B.; Freeman, R.D.; Bax, M. Evidence supporting the use of melatonin in short gestation infants. J. Pineal Res 2007, 42, 22–27. [Google Scholar]
- Gitto, E.; Reiter, R.J.; Cordaro, S.P.; La Rosa, M.; Chiurazzi, P.; Trimarchi, G.; Gitto, P.; Calabro, M.P.; Barberi, I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: Beneficial effects of melatonin. Am. J. Perinatol 2004, 21, 209–216. [Google Scholar]
- Gitto, E.; Reiter, R.J.; Amodio, A.; Romeo, C.; Cuzzocrea, E.; Sabatino, G.; Buonocore, G.; Cordaro, V.; Trimarchi, G.; Barberi, I. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J. Pineal Res 2004, 36, 250–255. [Google Scholar]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal. Neonatal Med 2007, 12, 287–295. [Google Scholar]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M.; et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013, 136, 90–105. [Google Scholar]
- Waldhauser, F.; Waldhauser, M.; Lieberman, H.R.; Deng, M.H.; Lynch, H.J.; Wurtman, R.J. Bioavailability of oral melatonin in humans. Neuroendocrinology 1984, 39, 307–313. [Google Scholar]
- Aldhous, M.; Franey, C.; Wright, J.; Arendt, J. Plasma concentrations of melatonin in man following oral absorption of different preparations. Br. J. Clin. Pharmacol 1985, 19, 517–521. [Google Scholar]
- Lane, E.A.; Moss, H.B. Pharmacokinetics of melatonin in man: First pass hepatic metabolism. J. Clin. Endocrinol. MeTable 1985, 61, 1214–1216. [Google Scholar]
- Merchant, N.M.; Azzopardi, D.V.; Hawwa, A.F.; McElnay, J.C.; Middleton, B.; Arendt, J.; Arichi, T.; Gressens, P.; Edwards, A.D. Pharmacokinetics of Melatonin in Preterm Infants. Br. J. Clin. Pharmacol. 2013. [Google Scholar] [CrossRef]

| Target | Effect | References |
|---|---|---|
| Brain Protection | ||
| Infarct volume | ↓ | [34–36] |
| Sensorimotor asymmetry | ↓ | [34] |
| Learning deficits | ↓ | [34] |
| Morphologically well preserved neurons | ↑ | [33,40,41] |
| GFAP expression | ↓ | [33] |
| MBP expression | ↑ | [33,50–52] |
| Antioxidant | ||
| Lipid peroxidation and MDA production | ↓ | [56–58] |
| Iso- and neuroprostanes and neurofurans | ↓ | [62–64] |
| Protein oxidation | ↓ | [65] |
| Catalase’s activity | → | [57] |
| Hydroxyl formation | ↓ | [56] |
| Nitrite/nitrate levels | ↓ | [58] |
| Anti-apoptotic | ||
| Cytochrome c release | ↓ | [32,67,68] |
| Caspase-1 and Caspase-3 activation | ↓ | [32,67,69–73,88,89] |
| Bcl-xL and Bcl-2 expression | ↑ | [70,71,74,75,89] |
| Bax expression | ↓ | [71] |
| Poly-ADP-ribose-polymerase cleavage | ↓ | [72] |
| Mitochondrial transition pore opening | ↓ | [67,69] |
| TUNEL-positive cells/DNA breaks | ↓ | [31–33,35,36,64,72,75–78] |
| Cytosolic calcium concentrations | ↓ | [81,82] |
| Oxidative mitochondria damage | ↓ | [87] |
| Mitochondrial respiratory activity | → | [40,41] |
| Oxidative stress | ↓ | [40,41] |
| Fractin levels | ↓ | [88] |
| Bcl-2/Bax ratio | ↑ | [89] |
| MAP kinase, JNK1/2 and ERK 1/2 | → | [31,72,90] |
| Bad dephosphorylation | ↓ | [31] |
| Anti-inflammatory | ||
| Interleukin-6, Interleukin-8 and Tumor Necrosis Factor- α | ↓ | [94–100,102,122,123,125,126] |
| 5-lipoxygenase and Cyclooxyenase-2 | ↓ | [101–103,127] |
| Prostaglandin | ↓ | [104,106] |
| NO, nNOS, iNOS and VEGF | ↓ | [107–112,124,128] |
| Macrophage infiltration | ↓ | [88] |
| ED1 positive cells | ↓ | [63] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alonso-Alconada, D.; Álvarez, A.; Arteaga, O.; Martínez-Ibargüen, A.; Hilario, E. Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia. Int. J. Mol. Sci. 2013, 14, 9379-9395. https://doi.org/10.3390/ijms14059379
Alonso-Alconada D, Álvarez A, Arteaga O, Martínez-Ibargüen A, Hilario E. Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia. International Journal of Molecular Sciences. 2013; 14(5):9379-9395. https://doi.org/10.3390/ijms14059379
Chicago/Turabian StyleAlonso-Alconada, Daniel, Antonia Álvarez, Olatz Arteaga, Agustín Martínez-Ibargüen, and Enrique Hilario. 2013. "Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia" International Journal of Molecular Sciences 14, no. 5: 9379-9395. https://doi.org/10.3390/ijms14059379
APA StyleAlonso-Alconada, D., Álvarez, A., Arteaga, O., Martínez-Ibargüen, A., & Hilario, E. (2013). Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia. International Journal of Molecular Sciences, 14(5), 9379-9395. https://doi.org/10.3390/ijms14059379