CD44 Is Associated with the Aggressive Phenotype of Nasopharyngeal Carcinoma through Redox Regulation
Abstract
:1. Introduction
2. Results
2.1. NPC Sphere-Derived Cells Show Stem-Like Properties and Chemotherapeutic Resistance, and Express the Mesenchymal Phenotype
2.2. Stemness Gene, Drug-Resistant Gene, and EMT Marker Expression in NPC Sphere-Derived Cells
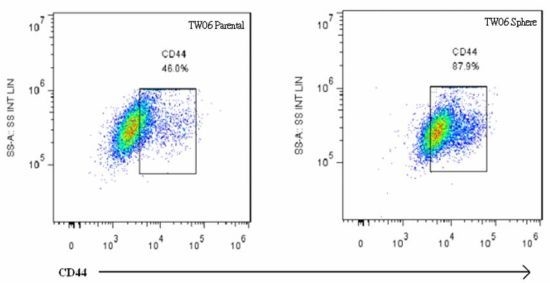
2.3. EMT Marker Expression Is Associated with CD44 Expression in NPC Sphere-Derived Cells
2.4. Redox Status in NPC Sphere-Derived Cells Is Associated with CD44 and EMT Marker Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.1.1. Parental Monolayer Cell Culture
4.1.2. Nonadherent Culture
4.2. Soft Agar Clonogenic Assay
4.3. Evaluation of Cell Viability Using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
4.4. Cell Invasion Analysis
4.5. Evaluation of Gene Expression using the Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
- GAPDH, Forward: 5′-ACGGGAAGCTCACTGGCATGG-3′; Reverse: 5′-GGTCCACCACCCTGTTGCTGTA-3′
- Nanog, Forward: 5′-ATTCAGGACAGCCCTGATTCTTC-3′; Reverse: 5′-TTTTTGCGACACTCTTCTCTGC-3′
- Oct-4, Forward: 5′-GTGGAGAGCAACTCCGATG-3′; Reverse: 5′-TGCTCCAGCTTCTCCTTCTC-3′
- MDR-1, Forward: 5′-TGGCAAAGAAATAAAGCGACTGA-3′; Reverse: 5′-CAGGATGGGCTCCTGGG-3′
- ABCG2, Forward: 5′-CATGTACTGGCGAAGAATATTTGGT-3′; Reverse: 5′-CACGTGATTCTTCCACAAGCC-3′
- N-Cad, Forward: 5′-AGGGTGGACGTCATTGTAGC-3′; Reverse: 5′-CTGTTGGGGTCTGTCAGGAT-3′
- Vim, Forward: 5′-GAGAACTTTGCCGTTGAAGC-3′; Reverse: 5′-GCTTCCTGTAGGTGGCAATC-3′
- Snail, Forward: 5′-CTTCCAGCAGCCCTACGAC-3′; Reverse: 5′-CGGTGGGGTTGAGGATCT-3′
- Gss, Forward: 5′-CCTGCTAGTGGATGCTGTCA-3′; Reverse: 5′-TCATCCTGTTTGATGGTGCT-3′
- GCLc, Forward: 5′-GTCTTCAGGTGACATTCCAAGC-3′; Reverse: 5′-TGTTCTTCAGGGGCTCCAGTC-3′
- GCLm, Forward: 5′-CTGCTAAACTGTTCATTGTAGG-3′; Reverse: 5′-CTATGGGTTTTACCTGTG-3′
4.6. Determination of Intracellular ROS Levels and Reduced Glutathione (GSH) Levels
4.7. Flow Cytometry for Antibody Analysis
4.8. CD44 siRNA Knockdown
4.9. Statistical Analysis
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Gu, A.D.; Zeng, M.S.; Qian, C.N. The criteria to confirm the role of epstein-barr virus in nasopharyngeal carcinoma initiation. Int. J. Mol. Sci 2012, 13, 13737–13747. [Google Scholar]
- Lo, K.W.; To, K.F.; Huang, D.P. Focus on nasopharyngeal carcinoma. Cancer Cell 2004, 5, 423–428. [Google Scholar]
- Ksiazkiewicz, M.; Markiewicz, A.; Zaczek, A.J. Epithelial-mesenchymal transition: A hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology 2012, 79, 195–208. [Google Scholar]
- Hollier, B.G.; Evans, K.; Mani, S.A. The epithelial-to-mesenchymal transition and cancer stem cells: A coalition against cancer therapies. J. Mammary Gland Biol. Neoplasia 2009, 14, 29–43. [Google Scholar]
- Guo, D.; Xu, B.L.; Zhang, X.H.; Dong, M.M. Cancer stem-like side population cells in the human nasopharyngeal carcinoma cell line cne-2 possess epithelial mesenchymal transition properties in association with metastasis. Oncol. Rep 2012, 28, 241–247. [Google Scholar]
- Su, J.; Xu, X.H.; Huang, Q.; Lu, M.Q.; Li, D.J.; Xue, F.; Yi, F.; Ren, J.H.; Wu, Y.P. Identification of cancer stem-like CD44+ cells in human nasopharyngeal carcinoma cell line. Arch. Med. Res 2011, 42, 15–21. [Google Scholar]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 2008, 68, 1777–1785. [Google Scholar]
- Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett 2008, 266, 53–59. [Google Scholar]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar]
- Vera-Ramirez, L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C.; Ramirez-Tortosa, C.L.; Granados-Principal, S.; Lorente, J.A.; Quiles, J.L. Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells. Biological bases to develop oxidative-based therapies. Crit. Rev. Oncol. Hematol 2011, 80, 347–368. [Google Scholar]
- Cannito, S.; Novo, E.; Compagnone, A.; Valfre di Bonzo, L.; Busletta, C.; Zamara, E.; Paternostro, C.; Povero, D.; Bandino, A.; Bozzo, F.; et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 2008, 29, 2267–2278. [Google Scholar]
- Wang, Z.; Li, Y.; Sarkar, F.H. Signaling mechanism(s) of reactive oxygen species in Epithelial-Mesenchymal Transition reminiscent of cancer stem cells in tumor progression. Curr. Stem. Cell Res. Ther 2010, 5, 74–80. [Google Scholar]
- Chen, C.; Zimmermann, M.; Tinhofer, I.; Kaufmann, A.M.; Albers, A.E. Epithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinoma. Cancer Lett. 2012. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Wang, Z.; Sarkar, F.H. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins? Cancers 2011, 3, 716–729. [Google Scholar]
- Luo, W.; Fang, W.; Li, S.; Yao, K. Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int. J. Cancer 2012, 131, 1863–1873. [Google Scholar]
- Bensouda, Y.; Kaikani, W.; Ahbeddou, N.; Rahhali, R.; Jabri, M.; Mrabti, H.; Boussen, H.; Errihani, H. Treatment for metastatic nasopharyngeal carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis 2011, 128, 79–85. [Google Scholar]
- Qiu, X.; Wang, Z.; Li, Y.; Miao, Y.; Ren, Y.; Luan, Y. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett 2012, 323, 161–170. [Google Scholar]
- Rybak, A.P.; He, L.; Kapoor, A.; Cutz, J.C.; Tang, D. Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim. Biophys. Acta 2011, 1813, 683–694. [Google Scholar]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 2009, 69, 5820–5828. [Google Scholar]
- Marchini, S.; Fruscio, R.; Clivio, L.; Beltrame, L.; Porcu, L.; Nerini, I.F.; Cavalieri, D.; Chiorino, G.; Cattoretti, G.; Mangioni, C.; et al. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur. J. Cancer 2013, 49, 520–530. [Google Scholar]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci 2005, 10, 1881–1896. [Google Scholar]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 2006, 25, 695–705. [Google Scholar]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar]
- Ween, M.P.; Oehler, M.K.; Ricciardelli, C. Role of Versican, hyaluronan and CD44 in ovarian cancer metastasis. Int. J. Mol. Sci 2011, 12, 1009–1029. [Google Scholar]
- Kawakami, S.; Kageyama, Y.; Fujii, Y.; Kihara, K.; Oshima, H. Inhibitory effect of N-acetylcysteine on invasion and MMP-9 production of T24 human bladder cancer cells. Anticancer Res 2001, 21, 213–219. [Google Scholar]
- Zahid, M.; Saeed, M.; Beseler, C.; Rogan, E.G.; Cavalieri, E.L. Resveratrol and N-acetylcysteine block the cancer-initiating step in MCF-10F cells. Free Radic. Biol. Med 2011, 50, 78–85. [Google Scholar]
- Lun, S.W.; Cheung, S.T.; Cheung, P.F.; To, K.F.; Woo, J.K.; Choy, K.W.; Chow, C.; Cheung, C.C.; Chung, G.T.; Cheng, A.S.; et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PLoS One 2012, 7, e52426. [Google Scholar]
- Shi, Y.; Tian, Y.; Zhou, Y.Q.; Ju, J.Y.; Qu, L.; Chen, S.L.; Xiang, Z.G.; Liu, Y.; Zhu, L.P. Inhibition of malignant activities of nasopharyngeal carcinoma cells with high expression of CD44 by siRNA. Oncol. Rep. 2007, 18, 397–403. [Google Scholar]
- Jin, H.; Zhao, H.; Chen, X.; Ma, L.; Huang, X.; Ye, H.; Cai, J. An easy method to detect the kinetics of CD44 antibody and its receptors on B16 cells using atomic force microscopy. Mol. Biol. Rep 2011, 38, 4495–4500. [Google Scholar]
- Geng, S.; Guo, Y.; Wang, Q.; Li, L.; Wang, J. Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma. Arch. Dermatol. Res 2013, 305, 35–47. [Google Scholar]
- Li, C.; Lee, C.J.; Simeone, D.M. Identification of human pancreatic cancer stem cells. Methods Mol. Biol 2009, 568, 161–173. [Google Scholar]
- Okamoto, A.; Chikamatsu, K.; Sakakura, K.; Hatsushika, K.; Takahashi, G.; Masuyama, K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol 2009, 45, 633–639. [Google Scholar]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 2008, 68, 4311–4320. [Google Scholar]
- Liu, C.M.; Chang, C.H.; Yu, C.H.; Hsu, C.C.; Huang, L.L. Hyaluronan substratum induces multidrug resistance in human mesenchymal stem cells via CD44 signaling. Cell Tissue Res 2009, 336, 465–475. [Google Scholar]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar]
- Nagano, O.; Okazaki, S.; Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 2013. [Google Scholar] [CrossRef]
- Tamada, M.; Nagano, O.; Tateyama, S.; Ohmura, M.; Yae, T.; Ishimoto, T.; Sugihara, E.; Onishi, N.; Yamamoto, T.; Yanagawa, H.; et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res 2012, 72, 1438–1448. [Google Scholar]
- Wang, S.J.; Wong, G.; de Heer, A.M.; Xia, W.; Bourguignon, L.Y. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 2009, 119, 1518–1530. [Google Scholar]
- Wu, G.; Zhou, Y.; Li, T.; Guo, J.; Zhou, Z. Immunohistochemical levels of matrix metalloproteinase-2 and CD44 variant 6 protein in the diagnosis and lateral cervical lymph node metastasis of papillary thyroid carcinoma. J. Int. Med. Res. 2013. [Google Scholar] [CrossRef]








© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, C.-H.; Hung, P.-H.; Chen, Y.-J. CD44 Is Associated with the Aggressive Phenotype of Nasopharyngeal Carcinoma through Redox Regulation. Int. J. Mol. Sci. 2013, 14, 13266-13281. https://doi.org/10.3390/ijms140713266
Lin C-H, Hung P-H, Chen Y-J. CD44 Is Associated with the Aggressive Phenotype of Nasopharyngeal Carcinoma through Redox Regulation. International Journal of Molecular Sciences. 2013; 14(7):13266-13281. https://doi.org/10.3390/ijms140713266
Chicago/Turabian StyleLin, Chien-Hung, Peir-Haur Hung, and Yann-Jang Chen. 2013. "CD44 Is Associated with the Aggressive Phenotype of Nasopharyngeal Carcinoma through Redox Regulation" International Journal of Molecular Sciences 14, no. 7: 13266-13281. https://doi.org/10.3390/ijms140713266
APA StyleLin, C. -H., Hung, P. -H., & Chen, Y. -J. (2013). CD44 Is Associated with the Aggressive Phenotype of Nasopharyngeal Carcinoma through Redox Regulation. International Journal of Molecular Sciences, 14(7), 13266-13281. https://doi.org/10.3390/ijms140713266