Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet
Abstract
:1. Introduction
2. Results and Discussion
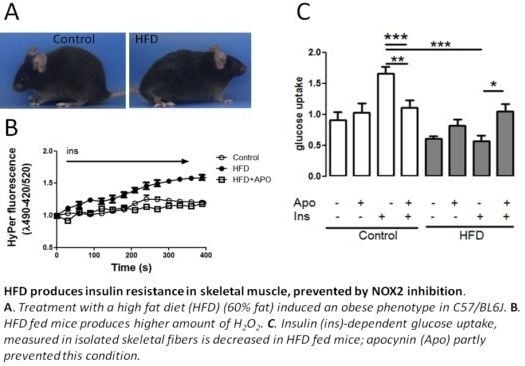
2.1. Establishing an Insulin Resistance Model
2.2. H2O2 Generation Is Higher in Muscle Fibers from High-Fat Diet Mice
2.3. Skeletal Muscle GSH Content in Insulin-Resistant Mice
2.4. Skeletal Muscle NOX2 Expression in Insulin-Resistant Mice
2.5. Apocynin in the Diet Prevents HFD-Induced Insulin Resistance in Mice
3. Experimental Section
3.1. Animals
3.2. Biochemical Determinations
3.3. Single-Cell Fluorescent 2-NBDG Uptake Assay
3.4. Fibers Transfection and H2O2 Measurement
3.5. Glutathione (GSH) Measurement
3.6. Western Blot Analysis
3.7. RT-PCR
3.8. Statistics
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol 2002, 90, 11G–18G. [Google Scholar]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef]
- Robinson, K.A.; Buse, M.G. Mechanisms of high-glucose/insulin-mediated desensitization of acute insulin-stimulated glucose transport and Akt activation. Am. J. Physiol. Endocrinol. Metab 2008, 294, E870–E881. [Google Scholar]
- Haque, A.; Andersen, J.N.; Salmeen, A.; Barford, D.; Tonks, N.K. Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell 2011, 147, 185–198. [Google Scholar]
- Carvalho-Filho, M.A.; Ueno, M.; Hirabara, S.M.; Seabra, A.B.; Carvalheira, J.B.; de Oliveira, M.G.; Velloso, L.A.; Curi, R.; Saad, M.J. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: A novel mechanism of insulin resistance. Diabetes 2005, 54, 959–967. [Google Scholar]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal 2008, 10, 1941–1988. [Google Scholar]
- Kang, S.; Kang, J.; Kwon, H.; Frueh, D.; Yoo, S.H.; Wagner, G.; Park, S. Effects of redox potential and Ca2+ on the inositol 1,4,5-trisphosphate receptor L3-1 loop region: Implications for receptor regulation. J. Biol. Chem 2008, 283, 25567–25575. [Google Scholar]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulere, L.; Lagarde, M.; Soulage, C.O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal 2005, 7, 1040–1052. [Google Scholar]
- Espinosa, A.; Garcia, A.; Hartel, S.; Hidalgo, C.; Jaimovich, E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J. Biol. Chem 2009, 284, 2568–2575. [Google Scholar]
- Osorio-Fuentealba, C.; Contreras-Ferrat, A.E.; Altamirano, F.; Espinosa, A.; Li, Q.; Niu, W.; Lavandero, S.; Klip, A.; Jaimovich, E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kgamma-Akt-AS160 in skeletal muscle cells. Diabetes 2012, 2012. [Google Scholar] [CrossRef]
- Dohm, G.L.; Tapscott, E.B.; Pories, W.J.; Dabbs, D.J.; Flickinger, E.G.; Meelheim, D.; Fushiki, T.; Atkinson, S.M.; Elton, C.W.; Caro, J.F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J. Clin. Invest 1988, 82, 486–494. [Google Scholar]
- Shortreed, K.E.; Krause, M.P.; Huang, J.H.; Dhanani, D.; Moradi, J.; Ceddia, R.B.; Hawke, T.J. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS One 2009, 4, e7293. [Google Scholar]
- Espinosa, A.; Leiva, A.; Pena, M.; Muller, M.; Debandi, A.; Hidalgo, C.; Carrasco, M.A.; Jaimovich, E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J. Cell Physiol 2006, 209, 379–388. [Google Scholar]
- DiFranco, M.; Quinonez, M.; Capote, J.; Vergara, J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J. Vis. Exp. 2009, 32. [Google Scholar] [CrossRef]
- Mofarrahi, M.; Brandes, R.P.; Gorlach, A.; Hanze, J.; Terada, L.S.; Quinn, M.T.; Mayaki, D.; Petrof, B.; Hussain, S.N. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid. Redox Signal 2008, 10, 559–574. [Google Scholar]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev 2007, 87, 245–313. [Google Scholar]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev 2009, 89, 27–71. [Google Scholar]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Matsushima, S.; Inoue, N.; Ohta, Y.; Hamaguchi, S.; Sobirin, M.A.; Ono, T.; Suga, T.; et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H1069–H1077. [Google Scholar]
- Wong, Y.C.; Sim, M.K.; Lee, K.O. Des-aspartate-angiotensin-I and angiotensin IV improve glucose tolerance and insulin signalling in diet-induced hyperglycaemic mice. Biochem. Pharmacol 2011, 82, 1198–1208. [Google Scholar]
- Mahadev, K.; Motoshima, H.; Wu, X.; Ruddy, J.M.; Arnold, R.S.; Cheng, G.; Lambeth, J.D.; Goldstein, B.J. The NAD(P)H oxidase homolog NOX4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell Biol 2004, 24, 1844–1854. [Google Scholar]
- Diamond-Stanic, M.K.; Marchionne, E.M.; Teachey, M.K.; Durazo, D.E.; Kim, J.S.; Henriksen, E.J. Critical role of the transient activation of p38 MAPK in the etiology of skeletal muscle insulin resistance induced by low-level in vitro oxidant stress. Biochem. Biophys. Res. Commun 2011, 405, 439–444. [Google Scholar]
- Wei, Y.; Chen, K.; Whaley-Connell, A.T.; Stump, C.S.; Ibdah, J.A.; Sowers, J.R. Skeletal muscle insulin resistance: Role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol. Regul. Integr. Comp. Physiol 2008, 294, R673–R680. [Google Scholar]
- Galgani, J.E.; Nunez, B.; Videla, L.A. Vanillin suppresses Kupffer cell-related colloidal carbon-induced respiratory burst activity in isolated perfused rat liver: Anti-inflammatory implications. Food Funct 2012, 3, 1319–1323. [Google Scholar]
- Silver, A.E.; Beske, S.D.; Christou, D.D.; Donato, A.J.; Moreau, K.L.; Eskurza, I.; Gates, P.E.; Seals, D.R. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 2007, 115, 627–637. [Google Scholar]
- Graciano, M.F.; Santos, L.R.; Curi, R.; Carpinelli, A.R. NAD(P)H oxidase participates in the palmitate-induced superoxide production and insulin secretion by rat pancreatic islets. J. Cell Physiol 2011, 226, 1110–1117. [Google Scholar]
- Lambertucci, R.H.; Hirabara, S.M.; dos Silveira, L.R.; Levada-Pires, A.C.; Curi, R.; Pithon-Curi, T.C. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J. Cell Physiol 2008, 216, 796–804. [Google Scholar]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest 2004, 114, 1752–1761. [Google Scholar]
- Wang, H.J.; Pan, Y.X.; Wang, W.Z.; Zucker, I.H.; Wang, W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J. Appl. Physiol 2009, 107, 450–459. [Google Scholar]
- Valenzuela, R.; Espinosa, A.; Gonzalez-Manan, D.; D’Espessailles, A.; Fernandez, V.; Videla, L.A.; Tapia, G. N-3 long-chain polyunsaturated Fatty Acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS One 2012, 7, e46400. [Google Scholar]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.; et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008, 57, 1071–1077. [Google Scholar]
- Meng, R.; Zhu, D.L.; Bi, Y.; Yang, D.H.; Wang, Y.P. Anti-oxidative effect of apocynin on insulin resistance in high-fat diet mice. Ann. Clin. Lab. Sci 2011, 41, 236–243. [Google Scholar]
- Heumuller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schroder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009, 119, 573–581. [Google Scholar]
- Noland, R.C.; Woodlief, T.L.; Whitfield, B.R.; Manning, S.M.; Evans, J.R.; Dudek, R.W.; Lust, R.M.; Cortright, R.N. Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance. Am. J. Physiol. Endocrinol. Metab 2007, 293, E986–E1001. [Google Scholar]
- Image Processing and Analysis in Java; National Institutes of Health: Bethesda, MD, USA. Available online: http://rsbweb.nih.gov/ij/ (accessed on 2 December 2012).
- Wesseling, S.; Ishola, D.A., Jr; Joles, J.A.; Bluyssen, H.A.; Koomans, H.A.; Braam, B. Resistance to oxidative stress by chronic infusion of angiotensin II in mouse kidney is not mediated by the AT2 receptor. Am. J. Physiol. Renal. Physiol. 2005, 288, F1191–F1200. [Google Scholar]





© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Espinosa, A.; Campos, C.; Díaz-Vegas, A.; Galgani, J.E.; Juretic, N.; Osorio-Fuentealba, C.; Bucarey, J.L.; Tapia, G.; Valenzuela, R.; Contreras-Ferrat, A.; et al. Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet. Int. J. Mol. Sci. 2013, 14, 15740-15754. https://doi.org/10.3390/ijms140815740
Espinosa A, Campos C, Díaz-Vegas A, Galgani JE, Juretic N, Osorio-Fuentealba C, Bucarey JL, Tapia G, Valenzuela R, Contreras-Ferrat A, et al. Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet. International Journal of Molecular Sciences. 2013; 14(8):15740-15754. https://doi.org/10.3390/ijms140815740
Chicago/Turabian StyleEspinosa, Alejandra, Cristian Campos, Alexis Díaz-Vegas, José E. Galgani, Nevenka Juretic, César Osorio-Fuentealba, José L. Bucarey, Gladys Tapia, Rodrigo Valenzuela, Ariel Contreras-Ferrat, and et al. 2013. "Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet" International Journal of Molecular Sciences 14, no. 8: 15740-15754. https://doi.org/10.3390/ijms140815740
APA StyleEspinosa, A., Campos, C., Díaz-Vegas, A., Galgani, J. E., Juretic, N., Osorio-Fuentealba, C., Bucarey, J. L., Tapia, G., Valenzuela, R., Contreras-Ferrat, A., Llanos, P., & Jaimovich, E. (2013). Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet. International Journal of Molecular Sciences, 14(8), 15740-15754. https://doi.org/10.3390/ijms140815740