The Relationship between Dioxin Congeners in the Breast Milk of Vietnamese Women and Sister Chromatid Exchange
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics
2.2. SCE (Sister Chromatid Exchange) Frequency and Dioxin Concentration
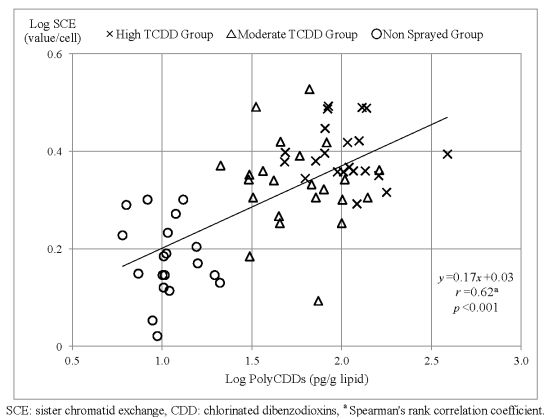
2.3. Single Regression Analysis of SCE Frequency and Dioxin Congeners
2.4. Multiple Regression Analysis of SCE Frequency and Regional Difference
2.5. Multiple Regression Analysis of SCE Frequency and Dioxin Congeners
2.6. Discussion
3. Experimental Section
3.1. Study Area and Population
3.2. Analysis of SCE
3.3. Analysis of Dioxin
3.4. Statistical Analysis
3.5. Ethical Approvals
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsT.K., Y.S., H.N. and N.N.H. designed the study. H.S., R.O., D.D.N., P.T.D., and L.H.T. collected data. K.T. and M.N. analyzed dioxins. H.H. and Y.S. analyzed SCE. H.S. performed statistical analysis and wrote the manuscript and T.K. supervised its analysis and editing the manuscript.
References
- Stellman, J.M.; Stellman, S.D.; Christian, R.; Weber, T.; Tomasallo, C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 2003, 422, 681–687. [Google Scholar]
- Kang, H.K.; Dalager, N.A.; Needham, L.L.; Patterson, D.G., Jr.; Lees, P.S.J.; Yates, K.; Matanoski, G.M. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am. J. Ind. Med 2006, 49, 875–884. [Google Scholar]
- Kim, J.S.; Lim, H.S.; Cho, S.I.; Cheong, H.K.; Lim, M.K. Impact of Agent Orange exposure among Korean Vietnam veterans. Ind. Health 2003, 41, 149–157. [Google Scholar]
- Michalek, J.E.; Ketchum, N.S.; Longnecker, M.P. Serum dioxin and hepatic abnormalities in veterans of operation ranch hand. Ann. Epidemiol 2001, 11, 304–311. [Google Scholar]
- Michalek, J.E.; Pavuk, M. Diabetes and cancer in veterans of operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. J. Occup. Environ. Med 2008, 50, 330–340. [Google Scholar]
- Schecter, A.; Furst, P.; Furst, C.; Papke, O.; Ball, M.; Ryan, J.J.; Cau, H.D.; Dai, L.C.; Quynh, H.T.; Cuong, H.Q.; et al. Chlorinated dioxins and dibenzofurans in human tissue from general populations: A selective review. Environ. Health Perspect 1994, 102 Suppl 1, 159–171. [Google Scholar]
- Schecter, A.; Le, C.D.; Thuy, L.T.B.; Quynh, H.T.; Minh, D.Q.; Cau, H.D.; Phiet, P.H.; Nguyen, N.T.; Constable, J.D.; Baughman, R. Agent Orange and the Vietnamese: The persistence of elevated dioxin levels in human tissues. Am. J. Public Health 1995, 85, 516–522. [Google Scholar]
- Schecter, A. A selective historical review of congener-specific human tissue measurements as sensitive and specific biomarkers of exposure to dioxins and related compounds. Environ. Health Perspect 1998, 106 Suppl 2, 737–742. [Google Scholar]
- Schecter, A.; Pavuk, M.; Päpke, O.; Ryan, J.J. Dioxin, dibenzofuran, and coplanar PCB levels in Laotian blood and milk from Agent Orange-sprayed and nonsprayed areas, 2001. J. Toxicol. Environ. Health Part A 2003, 66, 2067–2075. [Google Scholar]
- Kido, T.; Maruzeni, S.; Suzuki, H.; Odamae, Y.; Muranaka, M.; Naganuma, R.; Tawara, K.; Nishijo, M.; Nakagawa, H.; Hung, T.M.; et al. Five years studies on the long term effects of war agent orange/dioxin on human health in Vietnam. Organohalogen Compd 2007, 69, 572–575. [Google Scholar]
- Saito, K.; Nhu, D.D.; Suzuki, H.; Kido, T.; Naganuma, R.; Sakakibara, C.; Tawara, K.; Nishijo, M.; Nakagawa, H.; Kusama, K.; et al. Association between dioxin concentrations in breast milk and food group intake in Vietnam. Environ. Health Prev. Med 2009, 15, 48–56. [Google Scholar]
- Nhu, D.D.; Kido, T.; Naganuma, R.; Sawano, N.; Tawara, K.; Nishijo, M.; Nakagawa, H.; Hung, N.N.; Thom, L.T.H. A GIS study of dioxin contamination in a Vietnamese region sprayed with herbicide. Environ. Health Prev. Med 2009, 14, 353–360. [Google Scholar]
- Xinh, P.T.; Vu, H.A.; Man, H.V.; Tri, N.K.; Binh, N.T.; Nghia, H.; Trong, P.Q.; Binh, T.V.; Be, T.V.; Tokunaga, K.; et al. Unique secondary chromosomal abnormalities are frequently found in the chronic phase of chronic myeloid leukemia in southern Vietnam. Cancer Genet. Cytogenet 2006, 168, 59–68. [Google Scholar]
- Perry, P.; Evans, H.J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature 1975, 258, 121–125. [Google Scholar]
- Lundgren, K.; Collman, G.W.; Wang-Wuu, S.; Tiernan, T.; Taylor, M.; Thompson, C.L. Cytogenetic and chemical detection of human exposure to polyhalogenated aromatic hydrocarbons. Environ. Mol. Mutagen 1988, 11, 1–11. [Google Scholar]
- Rowland, R.E.; Edwards, L.A.; Podd, J.V. Elevated sister chromatid exchange frequencies in New Zealand Vietnam War veterans. Cytogenet. Genome Res 2007, 116, 248–251. [Google Scholar]
- Rifkind, A.B. CYP1A in TCDD toxicity and in physiology—With particular reference to CYP dependent arachidonic acid metabolism and other endogenous substrates. Drug Metab. Rev 2006, 38, 291–335. [Google Scholar]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev 1999, 13, 20–25. [Google Scholar]
- Horikawa, H.; Suzuki, H.; Naganuma, R.; Tawara, K.; Nishijo, H.; Nakagawa, H.; Hung, N.N.; Thom, L.T.H.; Dung, P.T.; et al. Relation between dioxins levels in human breast milk samples and SCE values among lactating females in a defoliants sprayed area in Vietnam. Organohalogen Compd 2008, 70, 646–649. [Google Scholar]
- Perucatti, A.; di Meo, G.P.; Albarella, S.; Ciotola, F.; Incarnato, D.; Caputi Jambrenghi, A.; Peretti, V.; Vonghia, G.; Iannuzzi, Z. Increased frequencies of both chromosome abnormalities and SCEs in two sheep flocks exposed to high dioxin levels during pasturage. Mutagen 2006, 21, 67–75. [Google Scholar]
- Di Meo, G.P.; Perucatti, A.; Genualdo, V.; Caputi Jambrenghi, A.; Rasero, A.; Nebbia, R.; Iannuzzi, L. Chromosome fragility in dairy cows exposed to dioxins and dioxin-like PSBs. Mutagen 2011, 26, 269–272. [Google Scholar]
- Nagayama, J.; Nagayama, M.; Iida, T.; Hirokawa, H.; Matsueda, T.; Masuda, Y. Effects of highly toxic organochlorine compounds retained in human body on induction of sister chromatid exchanges in cultured human lymphocytes. Chemosphere 1994, 29, 2349–2354. [Google Scholar]
- Nagayama, J.; Nagayama, M.; Haraguchi, K.; Kuroki, H.; Masuda, Y. Effect of 2,3,4,7,8-pentachlorodivenzofuran and its analogues on induction of sister chromatid exchanges in cultured human lymphocytes. Fukuoka Acta Med 1995, 86, 184–189. [Google Scholar]
- Iida, T.; Hirakawa, H.; Matsueda, T.; Takenaka, S.; Nagayama, J. Polychlorinated dibenzo-p-dioxins and related compounds in breast milk of Japanese primiparas and multiparas. Chemosphere 1999, 38, 2461–2466. [Google Scholar]
- Nagayama, J.; Nagayama, M.; Iida, T.; Hirokawa, H.; Matsueda, T.; Ohki, M.; Tsuji, H. Effects of donor age and contamination level of dioxin and related chemicals on frequency of sister chromatid exchanges in human lymphocytes cultured in vitro. Chemosphere 2001, 43, 845–849. [Google Scholar]
- Sardas, S.; Karahalil, B.; Akyol, D.; Kukner, S.; Karakaya, A.E. The effect of smoking on sister chromatid exchange rate of newborn infants born to smoking mothers. Mutat. Res. Genet. Toxicol 1995, 341, 249–253. [Google Scholar]
- Uehara, R.; Peng, G.; Nakamura, Y.; Matsuura, N.; Kondo, N.; Tada, H. Human milk survey for dioxins in the general population in Japan. Chemosphere 2006, 62, 1134–1141. [Google Scholar]
- Garaj-Vrhovac, V.; Zeljezic, D. Cytogenetic monitoring of Croatian population occupationally exposed to a complex mixture of pesticides. Toxicology 2001, 165, 153–162. [Google Scholar]
- Kido, T.; Naganuma, R.; Maruzeni, S.; Tawara, K.; Nishijo, M.; Nakagawa, H. An epidemiological study on health effects by dioxin in Vietnam (first report). The introduction of research and characteristic of the region. Proceedings of The 75th Annual Meeting of the Japanese Society of Hygiene, Niigata, Japan, 27–30, March 2005.
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; Vito, M.D.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World health organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compound. Toxicol. Sci 2006, 93, 223–241. [Google Scholar]
- DAC List of ODA Recipients Effective for Reporting on 2012 and 2013 Flows. Available online : http://www.oecd.org/dac/stats/DAC%20List%20used%20for%202012%20and%202013%20flows.pdf accessed on 25 April 2014.
- Milbrath, M.O.; Wenger, Y.; Chang, C.W.; Emond, C.; Garabrant, D.; Gillespie, B.W.; Jolliet, O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ. Health Perspect 2009, 117, 417–425. [Google Scholar]
- Kanda, N.; Kato, H. In vivo sister chromatid exchange in cells of various organs of the mouse. Chromosoma 1979, 74, 299–305. [Google Scholar]
- Goto, K.; Akematsu, T.; Shimazu, H.; Sugiyama, T. Simple differential Giemsa staining of sister chromatids after treatment with photosensitive dyes and exposure to light and the mechanism of staining. Chromosoma 1975, 53, 223–230. [Google Scholar]
- Patterson, D.G., Jr.; Holler, J.S.; Belser, W.T.; Boozer, E.L.; Lapeza, C.R.; Needham, L.L. Determination of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in human adipose tissue on whole-weight and lipid bases. Chemosphere 1987, 16, 935–936. [Google Scholar]
- Tawara, K.; Honda, R.; Nishijo, M.; Nakagawa, H. Pretreatment procedure of dioxin analysis for a small volume of human breast milk. J. Kanazawa Med. Univ 2003, 28, 17–25. [Google Scholar]
- Nishijo, M.; Tawara, K.; Nakagawa, H.; Honda, R.; Kido, T.; Nishijo, H.; Saito, S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in maternal breast milk and newborn head circumference. J. Expo. Sci. Environ. Epidemiol 2008, 18, 246–251. [Google Scholar]
- Tawara, K.; Nishijo, M.; Honda, R.; Maruzeni, S.; Seto, T.; Kido, T.; Saito, S.; Nakagawa, H. Effects of maternal dioxin exposure on newborn size at birth among Japanese mother-infant pairs. Environ. Health Prev. Med 2009, 14, 88–95. [Google Scholar]

| Itemes | High TCDD Group (1) | Moderate TCDD Group (2) | Non Sprayed Group (3) | p Value | Multiple Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 21 | n = 23 | n = 19 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||||
| Age [years] | 33.0 | (27.5–37.0) | 31.0 | (28.0–36.0) | 30.0 | (27.0–32.0) | 0.441 a | n.s d | n.s d | n.s d | |
| Height [cm] | 150.2 | (148.4–155.4) | 149.9 | (147.2–152.3) | 151.2 | (149.1–155.0) | 0.102 b | n.s e | n.s e | n.s e | |
| Weight [kg] | 44.0 | (43.0–46.0) | 41.0 | (40.0–47.0) | 45.0 | (42.0–48.0) | 0.122 a | n.s d | n.s d | n.s d | |
| BMI [kg/m2] | 19.3 | (18.3–20.6) | 18.7 | (17.7–20.9) | 19.2 | (18.3–20.5) | 0.792 b | n.s e | n.s e | n.s e | |
| Number of children | 2.0 | (1.0–2.5) | 2.0 | (2.0–3.0) | 1.0 | (1.0–2.0) | 0.079 a | n.s d | n.s d | n.s d | |
| Residence period [years] | 27.0 | (18.5–30.5) | 30.0 | (24.0–35.0) | 28.0 | (7.0–32.0) | 0.187 a | n.s d | n.s d | n.s d | |
| Family income [×104 VND/month] | 150 | (100–180) | 100 | (70–150) | 80 | (50–120) | 0.029 a | n.s d | *,d | n.s d | |
| Occupation | Farmer | 11 | (52.4%) | 22 | (95.7%) | 16 | (84.2%) | 0.002 c | **,f | n.s f | n.s f |
| Other | 10 | (47.6%) | 1 | (4.3%) | 3 | (15.8%) | - | - | - | - | |
| Smoking status | Smoker | 0 | (0.0%) | 3 | (13.0%) | 0 | (0.0%) | - | n.s f | - | n.s f |
| Non smoker | 21 | (100.0%) | 20 | (87.0%) | 19 | (100.0%) | - | - | - | - | |
| Items | High TCDD Group (1) | Moderate TCDD Group (2) | Non Sprayed Group (3) | p Value | Multiple Comparison | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 21 | n = 23 | n = 19 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||
| SCE [values/cell] | 2.40 | (2.28–2.72) | 2.19 | (2.00–2.35) | 1.48 | (1.35–1.71) | <0.001 a | *,c | ***,c | ***,c |
| Dioxins [pg/g lipid] | ||||||||||
| 2,3,7,8-TetraCDD | 1.39 | (1.15–2.07) | 0.57 | (0.31–0.74) | 0.55 | (0.48–0.66) | <0.001 a | ***,c | ***,c | n.s c |
| 1,2,3,7,8-PentaCDD | 3.62 | (2.85–5.57) | 1.68 | (1.25–2.66) | 1.25 | (1.00–1.35) | <0.001 a | ***,c | ***,c | *,c |
| 1,2,3,4,7,8-HexaCDD | 2.83 | (1.94–3.70) | 1.11 | (0.41–1.78) | 0.50 | (0.27–0.59) | <0.001 a | ***,c | ***,c | *,c |
| 1,2,3,6,7,8-HexaCDD | 9.05 | (7.63–13.03) | 4.87 | (3.18–7.50) | 1.20 | (0.75–1.61) | <0.001 a | ***,c | ***,c | ***,c |
| 1,2,3,7,8,9-HexaCDD | 2.53 | (1.89–4.33) | 1.01 | (0.68–2.02) | 0.48 | (0.24–0.58) | <0.001 a | ***,c | ***,c | ***,c |
| 1,2,3,4,6,7,8-HeptaCDD | 18.31 | (13.53–26.91) | 12.35 | (7.31–16.35) | 1.35 | (1.19–1.58) | <0.001 a | **,c | ***,c | ***,c |
| OctaCDD | 57.40 | (45.39–85.08) | 34.88 | (21.09–59.02) | 4.92 | (4.20–6.73) | <0.001 a | *c | ***,c | ***,c |
| Furans [pg/g lipid] | ||||||||||
| 2,3,7,8-TetraCDF | 0.60 | (0.50–0.76) | 0.51 | (0.32–0.69) | 1.01 | (0.80–1.34) | <0.001 a | n.s c | **,c | ***,c |
| 1,2,3,7,8-PentaCDF | 0.84 | (0.52–1.55) | 0.63 | (0.28–1.01) | 0.51 | (0.40–0.62) | <0.001 a | n.s c | *,c | n.s c |
| 2,3,4,7,8-PentaCDF | 6.35 | (5.01–11.17) | 3.07 | (2.16–5.15) | 2.70 | (1.69–3.39) | <0.001 a | ***,c | ***,c | n.s c |
| 1,2,3,4,7,8-HexaCDF | 17.84 | (14.75–35.03) | 9.87 | (5.37–16.39) | 1.58 | (0.64–2.80) | <0.001 b | **,d | **,d | ***,d |
| 1,2,3,6,7,8-HexaCDF | 9.70 | (7.94–21.92) | 5.54 | (4.03–10.49) | 0.95 | (0.60–1.72) | <0.001 a | *,c | ***,c | ***,c |
| 1,2,3,7,8,9-HexaCDF | 0.43 | (0.09–0.93) | 0.29 | (0.18–0.50) | 0.07 | (0.04–0.09) | <0.001 a | n.s c | **,c | ***,c |
| 2,3,4,6,7,8-HexaCDF | 1.56 | (1.13–2.67) | 0.81 | (0.35–1.52) | 0.36 | (0.23–0.43) | <0.001 a | **,c | ***,c | **,c |
| 1,2,3,4,6,7,8-HeptaCDF | 13.71 | (9.54–29.27) | 9.26 | (6.21–17.19) | 0.88 | (0.64–1.37) | <0.001 a | n.s c | ***,c | ***,c |
| 1,2,3,4,7,8,9-HeptaCDF | 2.31 | (1.33–4.16) | 1.37 | (0.50–2.59) | 0.08 | (0.04–0.12) | <0.001 a | *,c | ***,c | ***,c |
| OctaCDF | 0.21 | (0.08–1.06) | 0.22 | (0.11–0.52) | 0.07 | (0.04–0.16) | 0.004 a | n.s c | *,c | **,c |
| Total [pg/g lipid] | ||||||||||
| PolyCDDs | 107.80 | (80.62–132.62) | 58.38 | (33.33–82.43) | 10.34 | (8.84–13.12) | <0.001 b | ***,d | ***,d | ***,d |
| PolyCDFs | 58.33 | (43.97–102.50) | 31.27 | (21.71–59.26) | 9.24 | (4.94–12.70) | <0.001 a | **,c | ***,c | ***,c |
| PolyCDDs/Fs | 165.82 | (127.67–222.53) | 98.69 | (58.16–156.22) | 20.97 | (15.69–26.23) | <0.001 a | **,c | ***,c | ***,c |
| Total-TEQ [pg-TEQ/g lipid] | ||||||||||
| PolyCDDs-TEQ | 6.89 | (5.62–9.92) | 3.30 | (2.24–4.52) | 1.96 | (1.69–2.34) | <0.001 a | **,c | ***,c | ***,c |
| PolyCDFs-TEQ | 4.96 | (4.19–9.81) | 2.65 | (1.68–4.34) | 1.39 | (0.75–1.76) | <0.001 a | ***,c | ***,c | ***,c |
| PolyCDDs/Fs-TEQ | 12.62 | (10.00–17.11) | 5.90 | (4.00–9.25) | 3.40 | (2.51–4.10) | <0.001 a | ***,c | ***,c | ***,c |
| Dioxin Congers and Total Concentration | R | p Value |
|---|---|---|
| Dioxins | ||
| 2,3,7,8-TetraCDD | 0.45 | <0.001 |
| 1,2,3,7,8-PentaCDD | 0.49 | <0.001 |
| 1,2,3,4,7,8-HexaCDD | 0.54 | <0.001 |
| 1,2,3,6,7,8-HexaCDD | 0.63 | <0.001 |
| 1,2,3,7,8,9-HexaCDD | 0.54 | <0.001 |
| 1,2,3,4,6,7,8-HeptaCDD | 0.58 | <0.001 |
| OctaCDD | 0.61 | <0.001 |
| Furans | ||
| 2,3,7,8-TetraCDF | −0.42 | <0.001 |
| 1,2,3,7,8-PentaCDF | 0.04 | 0.782 |
| 2,3,4,7,8-PentaCDF | 0.38 | 0.002 |
| 1,2,3,4,7,8-HexaCDF | 0.55 | <0.001 |
| 1,2,3,6,7,8-HexaCDF | 0.55 | <0.001 |
| 1,2,3,7,8,9-HexaCDF | 0.20 | 0.110 |
| 2,3,4,6,7,8-HexaCDF | 0.38 | 0.002 |
| 1,2,3,4,6,7,8-HeptaCDF | 0.53 | <0.001 |
| 1,2,3,4,7,8,9-HeptaCDF | 0.56 | <0.001 |
| OctaCDF | 0.19 | 0.138 |
| Total | ||
| PolyCDDs | 0.62 | <0.001 |
| PolyCDFs | 0.52 | <0.001 |
| PolyCDDs/Fs | 0.57 | <0.001 |
| Total-TEQ | ||
| PolyCDDs-TEQ | 0.56 | <0.001 |
| PolyCDFs-TEQ | 0.50 | <0.001 |
| PolyCDDs/Fs-TEQ | 0.54 | <0.001 |
| Model | Standardized β | p Value | Adjusted R2 | p Value |
|---|---|---|---|---|
| Model 1 | ||||
| Sprayed area a | 0.73 | <0.001 | 0.49 | <0.001 |
| Model 2 | ||||
| High TCDD group b | 0.30 | 0.011 | 0.54 | <0.001 |
| Non sprayed group b | −0.58 | <0.001 | - | - |
| Dioxin Congers and Total Concentration | Standardized β | p Value | Adjusted R2 | p Value |
|---|---|---|---|---|
| Dioxins | ||||
| 2,3,7,8-TetraCDD | 0.34 | 0.016 | 0.09 | 0.076 |
| 1,2,3,7,8-PentaCDD | 0.42 | 0.001 | 0.16 | 0.014 |
| 1,2,3,4,7,8-HexaCDD | 0.40 | 0.002 | 0.15 | 0.020 |
| 1,2,3,6,7,8-HexaCDD | 0.60 | <0.001 | 0.35 | <0.001 |
| 1,2,3,7,8,9-HexaCDD | 0.48 | <0.001 | 0.23 | 0.002 |
| 1,2,3,4,6,7,8-HeptaCDD | 0.64 | <0.001 | 0.39 | <0.001 |
| OctaCDD | 0.65 | <0.001 | 0.37 | <0.001 |
| Furans | ||||
| 2,3,7,8-TetraCDF | −0.42 | 0.002 | 0.14 | 0.021 |
| 1,2,3,7,8-PentaCDF | −0.00 | 0.974 | −0.00 | 0.503 |
| 2,3,4,7,8-PentaCDF | 0.33 | 0.011 | 0.10 | 0.061 |
| 1,2,3,4,7,8-HexaCDF | 0.51 | <0.001 | 0.26 | <0.001 |
| 1,2,3,6,7,8-HexaCDF | 0.51 | <0.001 | 0.26 | <0.001 |
| 1,2,3,7,8,9-HexaCDF | 0.10 | 0.435 | 0.00 | 0.428 |
| 2,3,4,6,7,8-HexaCDF | 0.27 | 0.040 | 0.06 | 0.136 |
| 1,2,3,4,6,7,8-HeptaCDF | 0.54 | <0.001 | 0.29 | <0.001 |
| 1,2,3,4,7,8,9-HeptaCDF | 0.58 | <0.001 | 0.32 | <0.001 |
| OctaCDF | 0.09 | 0.529 | −0.00 | 0.453 |
| Total | ||||
| PolyCDDs | 0.64 | <0.001 | 0.38 | <0.001 |
| PolyCDFs | 0.47 | <0.001 | 0.22 | 0.003 |
| PolyCDDs/Fs | 0.59 | <0.001 | 0.32 | <0.001 |
| Total-TEQ | ||||
| PolyCDDs-TEQ | 0.50 | <0.001 | 0.23 | 0.002 |
| PolyCDFs-TEQ | 0.42 | <0.001 | 0.17 | 0.001 |
| PolyCDDs/Fs-TEQ | 0.48 | <0.001 | 0.21 | 0.003 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Suzuki, H.; Kido, T.; Okamoto, R.; Nhu, D.D.; Nishijo, M.; Nakagawa, H.; Tawara, K.; Horikawa, H.; Sato, Y.; Dung, P.T.; et al. The Relationship between Dioxin Congeners in the Breast Milk of Vietnamese Women and Sister Chromatid Exchange. Int. J. Mol. Sci. 2014, 15, 7485-7499. https://doi.org/10.3390/ijms15057485
Suzuki H, Kido T, Okamoto R, Nhu DD, Nishijo M, Nakagawa H, Tawara K, Horikawa H, Sato Y, Dung PT, et al. The Relationship between Dioxin Congeners in the Breast Milk of Vietnamese Women and Sister Chromatid Exchange. International Journal of Molecular Sciences. 2014; 15(5):7485-7499. https://doi.org/10.3390/ijms15057485
Chicago/Turabian StyleSuzuki, Hiroyuki, Teruhiko Kido, Rie Okamoto, Dang Duc Nhu, Muneko Nishijo, Hideaki Nakagawa, Kenji Tawara, Hiroaki Horikawa, Yuko Sato, Phung Tri Dung, and et al. 2014. "The Relationship between Dioxin Congeners in the Breast Milk of Vietnamese Women and Sister Chromatid Exchange" International Journal of Molecular Sciences 15, no. 5: 7485-7499. https://doi.org/10.3390/ijms15057485
APA StyleSuzuki, H., Kido, T., Okamoto, R., Nhu, D. D., Nishijo, M., Nakagawa, H., Tawara, K., Horikawa, H., Sato, Y., Dung, P. T., Thom, L. H., & Hung, N. N. (2014). The Relationship between Dioxin Congeners in the Breast Milk of Vietnamese Women and Sister Chromatid Exchange. International Journal of Molecular Sciences, 15(5), 7485-7499. https://doi.org/10.3390/ijms15057485