Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol—A Naturally Occurring Brominated Substance
Abstract
:1. Introduction

2. Results and Discussion
2.1. Fiber Morphology


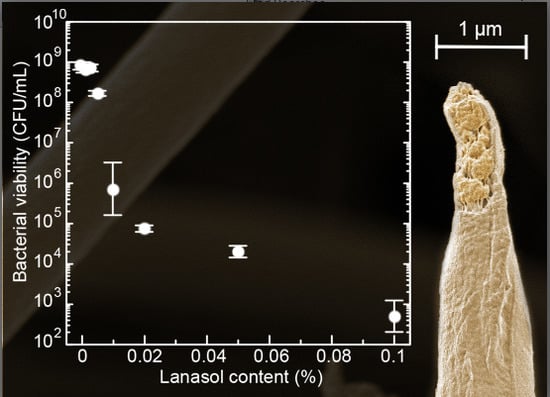
2.2. Antimicrobial Tests

2.3. Mechanical Properties

3. Experimental Section
3.1. Preparation of Fiber Solutions
3.2. Electrospinning and Collection of Fiber Mats
3.3. Bacterial Strain and Growth Conditions
3.4. Antimicrobial Tests
3.5. Tensile Testing of Fibers

3.6. Electron Microscopy
3.7. X-ray Diffraction (XRD)
3.8. Infrared (IR) Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver–NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.-H.; Ko, W.-K.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.-C.; et al. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar]
- Wang, H.; Cheng, M.; Hu, J.; Wang, C.; Xu, S.; Han, C.C. Preparation and optimization of silver nanoparticles embedded electrospun membrane for implant associated infections prevention. ACS Appl. Mater. Interfaces 2013, 5, 11014–11021. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly (l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Khalil, K.A.; Fouad, H.; Elsarnagawy, T.; Almajhdi, F.N. Preparation and characterization of electrospun plga/silver composite nanofibers for biomedical applications. Int. J. Electrochem. Sci. 2013, 8, 3483–3493. [Google Scholar]
- Schiffman, J.D.; Wang, Y.; Giannelis, E.P.; Elimelech, M. Biocidal activity of plasma modified electrospun polysulfone mats functionalized with polyethyleneimine-capped silver nanoparticles. Langmuir 2011, 27, 13159–13164. [Google Scholar] [CrossRef]
- Berger, T.J.; Spadaro, J.A.; Bierman, R.; Chapin, S.E.; Becker, R.O. Antifungal properties of electrically generated metallic ions. Antimicrob. Agents Chemother. 1976, 10, 856–860. [Google Scholar] [CrossRef]
- Xing, Z.-C.; Chae, W.-P.; Baek, J.-Y.; Choi, M.-J.; Jung, Y.; Kang, I.-K. In vitro assessment of antibacterial activity and cytocompatibility of silver-containing phbv nanofibrous scaffolds for tissue engineering. Biomacromolecules 2010, 11, 1248–1253. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, J.M.; Seo, K.S.; Jeong, Y.K.; Lee, H.B.; Khang, G. Characterization of degradation behavior for plga in various ph condition by simple liquid chromatography method. Biomed. Mater. Eng. 2005, 15, 279–288. [Google Scholar]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Globitza, K.W.; Stoffelen, H.; Murawski, U.; Bielaczek, J.; Egge, H. Antibiotics from algae. 9. Bromphenols from rhodemelaceae (author’s transl). Planta Med. 1974, 25, 105–114. [Google Scholar] [CrossRef]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wheelhouse, R.T.; Wright, C.W. In-vitro cytotoxic activities of the major bromophenols of the red alga polysiphonia lanosa and some novel synthetic isomers. J. Nat. Prod. 2004, 67, 1445–1449. [Google Scholar] [CrossRef]
- Allan, B. Closer to nature: New biomaterials and tissue engineering in ophthalmology. Br. J. Ophthalmol. 1999, 83, 1235–1240. [Google Scholar] [CrossRef]
- Amon, M.; Menapace, R. Cellular invasion on hydrogel and poly (methyl methacrylate) implants: An in vivo study. J. Cataract Refract. Surg. 1991, 17, 774–779. [Google Scholar] [CrossRef]
- Refojo, M.F. Artificial membranes for corneal surgery. J. Biomed. Mater. Res. 1969, 3, 333–347. [Google Scholar] [CrossRef]
- Shi, W.; Han, C.C. Dynamic competition between crystallization and phase separation at the growth interface of a PMMA/PEO blend. Macromolecules 2012, 45, 336–346. [Google Scholar] [CrossRef]
- Lodge, T.P.; Wood, E.R.; Haley, J.C. Two calorimetric glass transitions do not necessarily indicate immiscibility: The case of PEO/PMMA. J. Polym. Sci. B Polym. Phys. 2006, 44, 756–763. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Venkatachalapathy, P.D. Effect of casting solvent on the crystallization in PEO/PMMA blends. Polymer 1996, 37, 3749–3752. [Google Scholar] [CrossRef]
- Andersson, R.L.; Ström, V.; Gedde, U.W.; Mallon, P.E.; Hedenqvist, M.S.; Olsson, R.T. Micromechanics of ultra-toughened electrospun PMMA/PEO fibres as revealed by in-situ tensile testing in an electron microscope. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Stuart, B. Infrared Spectroscopy; Wiley Online Library: Hoboken, NJ, USA, 2005. [Google Scholar]
- Barreto, M.; Meyer, J.J.M. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (rhodophyta) and a visual exploration of its biofilm covering. South Afr. J. Bot. 2006, 72, 521–528. [Google Scholar] [CrossRef]
- Arunkumar, K.; Sivakumar, S.R.; Rengasamy, R. Review on bioactive potential in seaweeds (marine macroalgae): A special emphasis on bioactivity of seaweeds against plant pathogens. Asian J. Plant Sci. 2010, 9, 227–240. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Islam, M.M.; Masum, S.M.; Mahbub, K.R. In vitro antibacterial activity of shrimp chitosan against salmonela paratyphi and staphylococcus aureus. J. Bangladesh Chem. Soc. 2011, 24, 185–190. [Google Scholar]
- Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions; ASTM E2149; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2010.
- Antimicrobial Fabric Test; JIS L 1902; Japanese Industrial Standards (JIS): Tokyo, Japan, 2008.
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Van Rijen, M.; Bonten, M.; Wenzel, R.; Kluytmans, J. Mupirocin ointment for preventing staphylococcus aureus infections in nasal carriers. Cochrane Database Syst. Rev. 2008, 4. [Google Scholar] [CrossRef]
- Andersson, R.L.; Salajkova, M.; Mallon, P.E.; Berglund, L.A.; Hedenqvist, M.S.; Olsson, R.T. Micromechanical tensile testing of cellulose-reinforced electrospun fibers using a template transfer method (TTM). J. Polym. Environ. 2012, 20, 967–975. [Google Scholar] [CrossRef]
- Papkov, D.; Zou, Y.; Andalib, M.N.; Goponenko, A.; Cheng, S.Z.D.; Dzenis, Y.A. Simultaneously strong and tough ultrafine continuous nanofibers. ACS Nano 2013, 7, 3324–3331. [Google Scholar] [CrossRef]
- Determination of Antibacterial Activity of Textile Products; ISO 20743; International Organization for Standardization: Geneva, Switzerland, 2013.
- Antibacterial Finishes on Textile Materials; AATCC 100; American Association of Textile Chemists and Colorists (AATCC): Research Triangle Park, NC, USA, 2008.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Andersson, R.L.; Martínez-Abad, A.; Lagaron, J.M.; Gedde, U.W.; Mallon, P.E.; Olsson, R.T.; Hedenqvist, M.S. Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol—A Naturally Occurring Brominated Substance. Int. J. Mol. Sci. 2014, 15, 15912-15923. https://doi.org/10.3390/ijms150915912
Andersson RL, Martínez-Abad A, Lagaron JM, Gedde UW, Mallon PE, Olsson RT, Hedenqvist MS. Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol—A Naturally Occurring Brominated Substance. International Journal of Molecular Sciences. 2014; 15(9):15912-15923. https://doi.org/10.3390/ijms150915912
Chicago/Turabian StyleAndersson, Richard L., Antonio Martínez-Abad, José M. Lagaron, Ulf W. Gedde, Peter E. Mallon, Richard T. Olsson, and Mikael S. Hedenqvist. 2014. "Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol—A Naturally Occurring Brominated Substance" International Journal of Molecular Sciences 15, no. 9: 15912-15923. https://doi.org/10.3390/ijms150915912
APA StyleAndersson, R. L., Martínez-Abad, A., Lagaron, J. M., Gedde, U. W., Mallon, P. E., Olsson, R. T., & Hedenqvist, M. S. (2014). Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol—A Naturally Occurring Brominated Substance. International Journal of Molecular Sciences, 15(9), 15912-15923. https://doi.org/10.3390/ijms150915912