Understanding Melanocyte Stem Cells for Disease Modeling and Regenerative Medicine Applications
Abstract
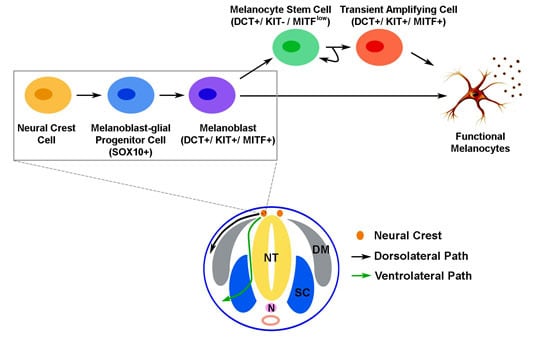
:1. Introduction
2. The Development of Melanocytic Lineage during Embryogenesis


3. Melanocyte Stem Cells (McSCs) in Hair Follicles
4. Interfollicular McSCs
5. McSCs in Phenotypic Abnormalities and Pathological Conditions
6. Melanocyte Differentiation in Pluripotent Stem Cells
7. Modeling Pathological Melanogenesis Using Pluripotent Stem Cell Techniques
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 1387. [Google Scholar] [CrossRef] [PubMed]
- Selleck, M.A.; Bronner-Fraser, M. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development 1995, 121, 525–538. [Google Scholar] [PubMed]
- Ernfors, P. Cellular origin and developmental mechanisms during the formation of skin melanocytes. Exp. Cell Res. 2010, 316, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Creuzet, S.; Couly, G.; Dupin, E. Neural crest cell plasticity and its limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Muller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 2009, 139, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.K.; Jordan, S.A.; Oshima, H.; Yoshida, H.; Osawa, M.; Moriyama, M.; Jackson, I.J.; Barrandon, Y.; Miyachi, Y.; Nishikawa, S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 2002, 416, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Kelley, P.M.; Kenyon, J.B.; Hoover, D. Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J. Med. Genet. 2000, 37, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Tassabehji, M.; Newton, V.E.; Read, A.P. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat. Genet. 1994, 8, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.J.; Erickson, C.A.; Takada, S.; Burrus, L.W. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev. Biol. 2001, 233, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Yasumoto, K.; Takada, R.; Takada, S.; Watanabe, K.; Udono, T.; Saito, H.; Takahashi, K.; Shibahara, S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000, 275, 14013–14016. [Google Scholar] [CrossRef] [PubMed]
- Raible, D.W.; Ragland, J.W. Reiterated Wnt and BMP signals in neural crest development. Semin. Cell Dev. Biol. 2005, 16, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.D.; Raible, D.W. Mechanisms for reaching the differentiated state: Insights from neural crest-derived melanocytes. Semin. Cell Dev. Biol. 2009, 20, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T. How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Res. 2011, 24, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Sommer, L. Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kunisada, T.; Kusakabe, M.; Nishikawa, S.; Nishikawa, S.I. Distinct stages of melanocyte differentiation revealed by anlaysis of nonuniform pigmentation patterns. Development 1996, 122, 1207–1214. [Google Scholar] [PubMed]
- Wehrle-Haller, B.; Weston, J.A. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development 1995, 121, 731–742. [Google Scholar] [PubMed]
- Tabone-Eglinger, S.; Wehrle-Haller, M.; Aebischer, N.; Jacquier, M.C.; Wehrle-Haller, B. Membrane-bound Kit ligand regulates melanocyte adhesion and survival, providing physical interaction with an intraepithelial niche. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 3738–3753. [Google Scholar] [CrossRef] [PubMed]
- Wehrle-Haller, B.; Meller, M.; Weston, J.A. Analysis of melanocyte precursors in Nf1 mutants reveals that MGF/KIT signaling promotes directed cell migration independent of its function in cell survival. Dev. Biol. 2001, 232, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Bondurand, N.; Pingault, V.; Goerich, D.E.; Lemort, N.; Sock, E.; Le Caignec, C.; Wegner, M.; Goossens, M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum. Mol. Genet. 2000, 9, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Potterf, S.B.; Furumura, M.; Dunn, K.J.; Arnheiter, H.; Pavan, W.J. Transcription factor hierarchy in Waardenburg syndrome: Regulation of MITF expression by SOX10 and PAX3. Hum. Genet. 2000, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takeda, K.; Yasumoto, K.; Udono, T.; Saito, H.; Ikeda, K.; Takasaka, T.; Takahashi, K.; Kobayashi, T.; Tachibana, M.; et al. Identification of a distal enhancer for the melanocyte-specific promoter of the MITF gene. Pigment Cell Res. Spons. Eur. Soc. Pigment Cell Res. Int. Pigment Cell Soc. 2002, 15, 201–211. [Google Scholar] [CrossRef]
- Tachibana, M.; Kobayashi, Y.; Matsushima, Y. Mouse models for four types of Waardenburg syndrome. Pigment Cell Res. Spons. Eur. Soc. Pigment Cell Res. Int. Pigment Cell Soc. 2003, 16, 448–454. [Google Scholar] [CrossRef]
- Adameyko, I.; Lallemend, F.; Furlan, A.; Zinin, N.; Aranda, S.; Kitambi, S.S.; Blanchart, A.; Favaro, R.; Nicolis, S.; Lubke, M.; et al. Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 2012, 139, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Erickson, C.A. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development 2009, 136, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, E.; Krispin, S.; Pfaltzgraff, E.R.; Klar, A.; Labosky, P.A.; Kalcheim, C. A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development 2013, 140, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Kizawa, K.; Hamada, K.; Cotsarelis, G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 2004, 72, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Myung, P.; Ito, M. Dissecting the bulge in hair regeneration. J. Clin. Investig. 2012, 122, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, P.; Takeo, M.; Chou, W.; Myung, P.; Bosenberg, M.; Chin, L.; Taketo, M.M.; Ito, M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 2011, 145, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 2011, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.K.; Suzuki, M.; Igras, V.; Du, J.; Lonning, S.; Miyachi, Y.; Roes, J.; Beermann, F.; Fisher, D.E. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 2010, 6, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Takeo, M.; Rabbani, P.; Hu, H.; Lee, W.; Chung, Y.R.; Carucci, J.; Overbeek, P.; Ito, M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat. Med. 2013, 19, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.B.; Koster, M.I.; Hoaglin, L.G.; Spoelstra, N.S.; Kechris, K.J.; Robinson, S.E.; Robinson, W.A.; Roop, D.R.; Norris, D.A.; Birlea, S.A. Narrow Band Ultraviolet B Treatment for Human Vitiligo Is Associated with Proliferation, Migration, and Differentiation of Melanocyte Precursors. J. Investig. Dermatol. 2015, 135, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.K. Melanocyte stem cells: A melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011, 24, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Hofmann, U.; Eichmuller, S.; Czarnetzki, B.M. Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br. J. Dermatol. 1994, 130, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; van der Veen, C.; Eichmuller, S.; Kopp, T.; Hagen, E.; Muller-Rover, S.; Hofmann, U. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Investig. Dermatol. 1998, 111, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.; Ugarte, K.; Chen, N.; Yachi, P.; Fuchs, E.; Boismenu, R.; Havran, W.L. A role for skin gammadelta T cells in wound repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.L.; Jameson, J.M.; Cauvi, G.; Havran, W.L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 2005, 6, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Honig, M.G.; Camilli, S.J.; Surineni, K.M.; Knight, B.K.; Hardin, H.M. The contributions of BMP4, positive guidance cues, and repulsive molecules to cutaneous nerve formation in the chick hindlimb. Dev. Biol. 2005, 282, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, Y.; Diaz-Balzac, C.A.; Ramirez-Suarez, N.J.; Attreed, M.; Tecle, E.; Desbois, M.; Kaprielian, Z.; Bulow, H.E. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell 2013, 155, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Woo, W.M.; Nagao, K.; Li, W.; Terunuma, A.; Mukouyama, Y.S.; Oro, A.E.; Vogel, J.C.; Brownell, I. Perivascular hair follicle stem cells associate with a venule annulus. J. Investig. Dermatol. 2013, 133, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.D.; Knolle, S.; Wells, K.L.; Liu, D.; Jackson, I.J.; Mort, R.L.; Headon, D.J. Maintenance of distinct melanocyte populations in the interfollicular epidermis. Pigment Cell Melanoma Res. 2015, 28, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Longley, B.J., Jr.; Morganroth, G.S.; Tyrrell, L.; Ding, T.G.; Anderson, D.M.; Williams, D.E.; Halaban, R. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N. Engl. J. Med. 1993, 328, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.R.; Whitaker-Menezes, D.; Longley, J.; Bender, J.; Murphy, G.F. Human dermal endothelial cells express membrane-associated mast cell growth factor. J. Investig. Dermatol. 1995, 104, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hayashi, S.; Shultz, L.D.; Yamamura, K.; Nishikawa, S.; Nishikawa, S.; Kunisada, T. Neural and skin cell-specific expression pattern conferred by steel factor regulatory sequence in transgenic mice. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1996, 207, 222–232. [Google Scholar]
- Kunisada, T.; Lu, S.Z.; Yoshida, H.; Nishikawa, S.; Nishikawa, S.; Mizoguchi, M.; Hayashi, S.; Tyrrell, L.; Williams, D.A.; Wang, X.; et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J. Exp. Med. 1998, 187, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, T.; Yoshida, H.; Yamazaki, H.; Miyamoto, A.; Hemmi, H.; Nishimura, E.; Shultz, L.D.; Nishikawa, S.; Hayashi, S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development 1998, 125, 2915–2923. [Google Scholar] [PubMed]
- Li, L.; Fukunaga-Kalabis, M.; Yu, H.; Xu, X.; Kong, J.; Lee, J.T.; Herlyn, M. Human dermal stem cells differentiate into functional epidermal melanocytes. J. Cell Sci. 2010, 123 Pt 6, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.; Tan, S.H.; Koh, W.L.; Chau, R.M.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013, 342, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Aoto, T.; Uhara, H.; Yamazaki, S.; Akutsu, H.; Umezawa, A.; Nakauchi, H.; Miyachi, Y.; Saida, T.; Nishimura, E.K. A melanocyte—Melanoma precursor niche in sweat glands of volar skin. Pigment Cell Melanoma Res. 2014, 27, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005, 307, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Budi, E.H.; Patterson, L.B.; Parichy, D.M. Post-embryonic nerve-associated precursors to adult pigment cells: Genetic requirements and dynamics of morphogenesis and differentiation. PLoS Genet. 2011, 7, e1002044. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Aoto, T.; Binh, N.T.; Okamoto, N.; Tanimura, S.; Wakayama, T.; Iseki, S.; Hara, E.; Masunaga, T.; Shimizu, H.; et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009, 137, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Aoto, T.; Mohri, Y.; Yokozeki, H.; Nishimura, E.K. Coupling of the radiosensitivity of melanocyte stem cells to their dormancy during the hair cycle. Pigment Cell Melanoma Res. 2014, 27, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, R.; Omidian, M.; Bagherani, N. Vitiligo: A review of the published work. J. Dermatol. 2011, 38, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Spritz, R.A. The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res. Spons. Eur. Soc. Pigment Cell Res. Int. Pigment Cell Soc. 2007, 20, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Kobayashi, T.; Moro, K.; Ohyama, M.; Adachi, T.; Kitashima, D.Y.; Ueha, S.; Horiuchi, K.; Tanizaki, H.; Kabashima, K.; et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012, 13, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Mihm, M.C., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.E.; Rosenbaum, L.E.; Bosenberg, M. Decoding melanoma metastasis. Cancers 2010, 3, 126–163. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Leishear, K.; Nguyen, T.K.; Finko, R.; Cai, K.; Fukunaga, M.; Li, L.; Brafford, P.A.; Kulp, A.N.; Xu, X.; et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells 2006, 24, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Hayashi, S.; Mizoguchi, M.; Yamazaki, H.; Kunisada, T. Derivation of melanocytes from embryonic stem cells in culture. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1999, 216, 450–458. [Google Scholar]
- Pla, P.; Alberti, C.; Solov'eva, O.; Pasdar, M.; Kunisada, T.; Larue, L. Ednrb2 orients cell migration towards the dorsolateral neural crest pathway and promotes melanocyte differentiation. Pigment Cell Res. Spons. Eur. Soc. Pigment Cell Res. Int. Pigment Cell Soc. 2005, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Nissan, X.; Larribere, L.; Saidani, M.; Hurbain, I.; Delevoye, C.; Feteira, J.; Lemaitre, G.; Peschanski, M.; Baldeschi, C. Functional melanocytes derived from human pluripotent stem cells engraft into pluristratified epidermis. Proc. Natl. Acad. Sci. USA 2011, 108, 14861–14866. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Imaizumi, Y.; Okada, Y.; Akamatsu, W.; Kuwahara, R.; Ohyama, M.; Amagai, M.; Matsuzaki, Y.; Yamanaka, S.; Okano, H.; et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS ONE 2011, 6, e16182. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Sabatini, K.; Liao, X.; Tran, H.T.; Lynch, C.L.; Morey, R.E.; Glenn-Pratola, V.; Boscolo, F.S.; Yang, Q.; Parast, M.M.; et al. Melanocytes derived from transgene-free human induced pluripotent stem cells. J. Investig. Dermatol. 2013, 133, 2104–2108. [Google Scholar] [CrossRef] [PubMed]
- Mica, Y.; Lee, G.; Chambers, S.M.; Tomishima, M.J.; Studer, L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013, 3, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Sviderskaya, E.V.; Wakeling, W.F.; Bennett, D.C. A cloned, immortal line of murine melanoblasts inducible to differentiate to melanocytes. Development 1995, 121, 1547–1557. [Google Scholar] [PubMed]
- Kawa, Y.; Ito, M.; Ono, H.; Asano, M.; Takano, N.; Ooka, S.; Watabe, H.; Hosaka, E.; Baba, T.; Kubota, Y.; et al. Stem cell factor and/or endothelin-3 dependent immortal melanoblast and melanocyte populations derived from mouse neural crest cells. Pigment Cell Res. Spons. Eur. Soc. Pigment Cell Res. Int. Pigment Cell Soc. 2000, 13 (Suppl. S8), 73–80. [Google Scholar] [CrossRef]
- Sviderskaya, E.V.; Hill, S.P.; Balachandar, D.; Barsh, G.S.; Bennett, D.C. Agouti signaling protein and other factors modulating differentiation and proliferation of immortal melanoblasts. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 221, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.L.; Donatien, P.D.; Smith, A.G.; Murphy, M.; Jones, M.K.; Herlyn, M.; Bennett, D.C.; Leonard, J.H.; Sturm, R.A. Human melanoblasts in culture: Expression of BRN2 and synergistic regulation by fibroblast growth factor-2, stem cell factor, and endothelin-3. J. Investig. Dermatol. 2003, 121, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.G.; Bin, B.H.; Choi, H.; Park, P.J.; Kang, H.H.; Lee, T.R. Novel method for isolating human melanoblasts from keratinocyte culture. Pigment Cell Melanoma Res. 2014, 27, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa-Torikai, S.; Osawa, M.; Nishikawa, S. Functional characterization of melanocyte stem cells in hair follicles. J. Investig. Dermatol. 2011, 131, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larribere, L.; Wu, H.; Novak, D.; Galach, M.; Bernhardt, M.; Orouji, E.; Weina, K.; Knappe, N.; Sachpekidis, C.; Umansky, L.; et al. NF1 loss induces senescence during human melanocyte differentiation in an iPSC-based model. Pigment Cell Melanoma Res. 2015, 28, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Maherali, N.; Kulalert, W.; Hochedlinger, K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J. Cell Sci. 2009, 122 Pt 19, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Mascarenhas, J.B.; Shea, C.R. Melanocytes, melanocyte stem cells, and melanoma stem cells. Clin. Dermatol. 2013, 31, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Kasemeier-Kulesa, J.; Kulesa, P.M.; Postovit, L.M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 2007, 7, 246–255. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mull, A.N.; Zolekar, A.; Wang, Y.-C. Understanding Melanocyte Stem Cells for Disease Modeling and Regenerative Medicine Applications. Int. J. Mol. Sci. 2015, 16, 30458-30469. https://doi.org/10.3390/ijms161226207
Mull AN, Zolekar A, Wang Y-C. Understanding Melanocyte Stem Cells for Disease Modeling and Regenerative Medicine Applications. International Journal of Molecular Sciences. 2015; 16(12):30458-30469. https://doi.org/10.3390/ijms161226207
Chicago/Turabian StyleMull, Amber N., Ashwini Zolekar, and Yu-Chieh Wang. 2015. "Understanding Melanocyte Stem Cells for Disease Modeling and Regenerative Medicine Applications" International Journal of Molecular Sciences 16, no. 12: 30458-30469. https://doi.org/10.3390/ijms161226207
APA StyleMull, A. N., Zolekar, A., & Wang, Y. -C. (2015). Understanding Melanocyte Stem Cells for Disease Modeling and Regenerative Medicine Applications. International Journal of Molecular Sciences, 16(12), 30458-30469. https://doi.org/10.3390/ijms161226207